


1. Keygesic-10
2. Magan
3. Magnesium Salicylate
4. Mobidin
5. Momentum (magnesium Salicylate)
1. 18917-95-8
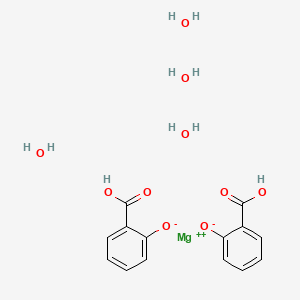
2. Magnesium Salicylate
3. Magnesium Salicylate Tetrahydrate
4. Backache Caplets
5. Bayer Select Backache
6. 41728cy7ux
7. Magnesium Salicylate (1:2), Tetrahydrate
8. 6150-94-3
9. Magnesiumsalicylatetetrahydrate
10. Chembl3989810
11. Dtxsid601015599
12. Magnesium Salicylate [inci]
13. Magnesium Salicylate [vandf]
14. Mfcd01766176
15. Magnesium Salicylate [usp-rs]
16. Magnesium Salicylate [who-dd]
17. Magnesium Salt Of 2-hydroxybenzoic Acid
18. Magnesium Salicylate [usp Monograph]
19. Magnesium 2-hydroxybenzoate Hydrate (1:2:4)
20. Magnesium Salicylate Tetrahydrate [mi]
21. Q20817210
22. Magnesium, Bis(2-(hydroxy-.kappa.o)benzoato-.kappa.o)-, Hydrate (1:4), (t-4)-
| Molecular Weight | 370.59 g/mol |
|---|---|
| Molecular Formula | C14H18MgO10 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 2 |
| Exact Mass | 370.0750385 g/mol |
| Monoisotopic Mass | 370.0750385 g/mol |
| Topological Polar Surface Area | 125 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 133 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 7 |
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Cyclooxygenase Inhibitors
Compounds or agents that combine with cyclooxygenase (PROSTAGLANDIN-ENDOPEROXIDE SYNTHASES) and thereby prevent its substrate-enzyme combination with arachidonic acid and the formation of eicosanoids, prostaglandins, and thromboxanes. (See all compounds classified as Cyclooxygenase Inhibitors.)