

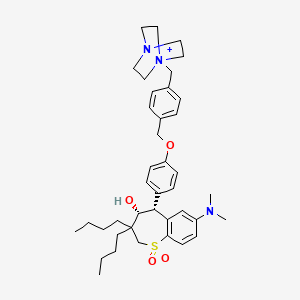

1. (4r,5r)-5-(4-((4-(4-aza-1-azoniabicyclo(2.2.2)octan-1-ylmethyl)phenyl)methoxy)phenyl)-3,3-dibutyl-7-(dimethylamino)-1,1-dioxo-4,5-dihydro-2h-16-benzothiepin-4-ol
2. Livmarli
1. Lopixibat
2. Maralixibat [usan]
3. 716313-53-0
4. Maralixibat Cation
5. Livmarli
6. Uyb6uof69l
7. Lum001 Cation
8. Lum-001 Cation
9. Chembl363392
10. Maralixibat (usan)
11. 4-aza-1-azoniabicyclo(2.2.2)octane, 1-((4-((4-((4r,5r)-3,3-dibutyl-7-(dimethylamino)-2,3,4,5-tetrahydro-4-hydroxy-1,1-dioxido-1-benzothiepin-5-yl)phenoxy)methyl)phenyl)methyl)-
12. Lopixibat Cation
13. Chembl17879
14. Lum 001
15. 1-(4-((4-((4r,5r)-3,3-dibutyl-7-(dimethylamino)-4-hydroxy-1,1-dioxido-2,3,4,5-tetrahydrobenzo[b]thiepin-5-yl)phenoxy)methyl)benzyl)-1,4-diazabicyclo[2.2.2]octan-1-ium
16. Unii-uyb6uof69l
17. Lopixibat (deleted Inn)
18. Maralixibat [who-dd]
19. Schembl10013954
20. Gtpl11708
21. Dtxsid001337103
22. Bdbm50140282
23. Compound 74 [pmid: 16134951]
24. D10951
25. Q27291331
26. (4r,5r)-5-[4-[[4-(4-aza-1-azoniabicyclo[2.2.2]octan-1-ylmethyl)phenyl]methoxy]phenyl]-3,3-dibutyl-7-(dimethylamino)-1,1-dioxo-4,5-dihydro-2h-1lambda6-benzothiepin-4-ol
27. 1-{4-[4-((4r,5r)-3,3-dibutyl-7-dimethylamino-4-hydroxy-1,1-dioxo-2,3,4,5-tetrahydro-1h-1lambda*6*-benzo[b]thiepin-5-yl)-phenoxymethyl]-benzyl}-4-aza-1-azonia-bicyclo[2.2.2]octane; Chloride
| Molecular Weight | 675.0 g/mol |
|---|---|
| Molecular Formula | C40H56N3O4S+ |
| XLogP3 | 7.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 13 |
| Exact Mass | 674.39915345 g/mol |
| Monoisotopic Mass | 674.39915345 g/mol |
| Topological Polar Surface Area | 78.5 Ų |
| Heavy Atom Count | 48 |
| Formal Charge | 1 |
| Complexity | 1080 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Maralixibat is indicated in the treatment of cholestatic pruritus in patients with Alagille syndrome who are at least 1 year old.
Maralixibat is indicated in the treatment of cholestatic pruritus in patients with Alagille syndrome who are at least 1 year old. It has a moderate duration of action as it is given once daily, and a wide therapeutic index as patients have safely tolerated single doses up to 18 times the normal dose. Patients should be counselled regarding the risks of liver test abnormalities, gastrointestinal adverse reactions, and fat-soluble vitamin deficiencies.
Absorption
Maralixibat is not extensively absorbed. A single 30 mg dose of maralixibat given under fasted conditions reached a median Tmax of 0.75 hours, with a mean Cmax of 1.65 1.10 ng/mL, and a mean AUClast of 3.43 2.13 h\*ng/mL. In pediatric patients given a dose of 380 g/kg, the highest serum concentration was 5.93 ng/mL, but was <0.25 ng/mL in the majority of patients.
Route of Elimination
A 5 mg radiolabelled dose of maralixibat is 73% eliminated in feces and 0.066% eliminated in urine. 94% of the dose recovered in the feces was as the unmetabolized parent compound. <3% of the total dose is metabolized.
Maralixibat metabolites have not been identified in plasma, however 3 minor metabolites have been recovered in the feces. The structure of these metabolites have not been defined in the literature.
The mean half life of maralixibat is 1.6 hours.
Patients with Alagille syndrome experience potentially debilitating pruritus. The exact mechanism of cholestatic pruritus in Alagille syndrome is not well defined, however it is correlated with elevated total serum bile acid concentrations. Enterohepatic circulation involves the synthesis of bile acid from cholesterol in the liver, conjugation with glycine or taurine, excretion into the duodenum, 95% resorption in the distal ileum through the ileal bile acid transporter (IBAT), return to the liver via the portal vein, and uptake into the liver by the sodium-dependent taurocholate co-transporting peptide (NTCP). It is important to note that unconjugated bile acids may freely diffuse across the intestinal mucosa or be transported across by other organic anion transporters. Maralixibat reversibly inhibits IBAT to decrease bile acid resorption in the ileum, leading to decreased resorption of bile acids in the distal ileum, increased elimination of bile acids in the feces, and decreased serum bile acids. The mechanism of action of maralixibat also leads to increased rates of diarrhea in patients. Under normal conditions, bile acids binding to the farnesoid X receptor (FXR) in the liver by via nuclear receptor small heterodimer partner (SHP) or in the ileum via fibroblast growth factor 19 (FGF19), triggers signal cascade that inhibits CYP7A1-mediated bile acid synthesis. Inhibition of IBAT by maralixibat, inhibits these negative feedback loops, leading to increased bile acid synthesis, and a reduction of low density lipoprotein cholesterol. In one clinical trial (NCT02057692), not all dose strengths were associated with a clinically significant difference between maralixibat and placebo.