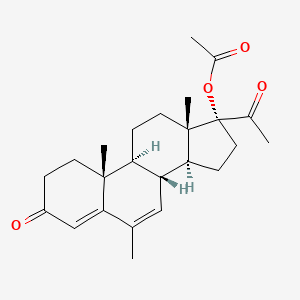

1. Acetate, Megestrol
2. Apo Megestrol
3. Apo-megestrol
4. Apomegestrol
5. Borea
6. Lin Megestrol
7. Lin-megestrol
8. Linmegestrol
9. Maygace
10. Megace
11. Megefren
12. Megestat
13. Megostat
14. Mestrel
15. Nu Megestrol
16. Nu-megestrol
17. Numegestrol
1. 595-33-5
2. Megace
3. Megace Es
4. Niagestin
5. Megestryl Acetate
6. Megeron
7. Megestat
8. Ovaban
9. Ovarid
10. Maygace
11. Pallace
12. Bdh 1298
13. Magestin
14. Sc 10363
15. Nsc-71423
16. Volidan
17. Sc10363
18. 17alpha-hydroxy-6-methylpregna-4,6-diene-3,20-dione Acetate
19. Megesterol Acetate
20. 17alpha-acetoxy-6-dehydro-6-methylprogesterone
21. 6-dehydro-6-methyl-17alpha-acetoxyprogesterone
22. 6-methyl-6-dehydro-17alpha-acetoxyprogesterone
23. 17-hydroxy-6-methylpregna-4,6-diene-3,20-dione 17-acetate
24. 17-acetoxy-6-methylpregna-4,6-diene-3,20-dione
25. Sc-10363
26. 17-(acetyloxy)-6-methylpregna-4,6-diene-3,20-dione
27. Bdh-1298
28. 6-methyl-17alpha-hydroxy-delta(sup 6)-progesterone Acetate
29. Tj2m0fr8es
30. [(8r,9s,10r,13s,14s,17r)-17-acetyl-6,10,13-trimethyl-3-oxo-2,8,9,11,12,14,15,16-octahydro-1h-cyclopenta[a]phenanthren-17-yl] Acetate
31. Pregna-4,6-diene-3,20-dione, 17-(acetyloxy)-6-methyl-
32. 6-methyl-3,20-dioxopregna-4,6-dien-17-yl Acetate
33. Megestrol 17.alpha.-acetate
34. Mls000028633
35. 17-hydroxy-6-methylpregna-4,6-diene-3,20-dione Acetate
36. Bdh1298
37. Smr000058691
38. Dsstox_cid_20683
39. Dsstox_rid_79539
40. Dsstox_gsid_40683
41. Megestil
42. 6-methyl-17alpha-acetoxypregna-4,6-diene-3,20-dione
43. Ovaban (veterinary)
44. 6-methyl-delta(sup 6)-dehydro-17alpha-acetoxyprogesterone
45. Megestrolacetate
46. 6-methyl-delta(sup 4,6)-pregnadien-17alpha-ol-3,20-dione Acetate
47. Megestrol Acetate [usan]
48. (8xi,9xi,10xi,13xi,14xi)-6-methyl-3,20-dioxopregna-4,6-dien-17-yl Acetate
49. Ccris 372
50. Pregna-4,6-diene-3,20-dione, 17-hydroxy-6-methyl-, Acetate
51. Megestrol-17-acetate
52. Einecs 209-864-5
53. Unii-tj2m0fr8es
54. Megestin
55. Megestrol Acetole [progestins]
56. Megestrol Acetate [usan:usp]
57. Megestrol Acetole
58. Megestrol-acetate
59. 17-alpha-acetoxy-6-dehydro-6-methylprogesterone
60. 6-dehydro-6-methyl-17-alpha-acetoxyprogesterone
61. 6-methyl-6-dehydro-17-alpha-acetoxyprogesterone
62. Ncgc00016516-01
63. (8r,9s,10r,13s,14s,17r)-17-acetyl-6,10,13-trimethyl-3-oxo-2,3,8,9,10,11,12,13,14,15,16,17-dodecahydro-1h-cyclopenta[a]phenanthren-17-yl Acetate
64. Cas-595-33-5
65. Megace (tn)
66. 17alpha-acetoxy-6-methylpregna-4,6-diene-3,20-dione
67. 6-methyl-17-alpha-acetoxypregna-4,6-diene-3,20-dione
68. 6-methyl-17-alpha-hydroxy-delta(sup 6)-progesterone Acetate
69. 6-methyl-6-dehydro-17.alpha.-acetylprogesterone
70. 6-methyl-delta(sup 6)-dehydro-17-alpha-acetoxyprogesterone
71. 6-methyl-delta4,6-pregnadien-17alpha-ol-3,20-dione Acetate
72. 17.alpha.-acetoxy-6-dehydro-6-methylprogesterone
73. 6-dehydro-6-methyl-17.alpha.-acetoxyprogesterone
74. 6-methyl-6-dehydro-17.alpha.-acetoxyprogesterone
75. Megestrol Acetate (usp)
76. Opera_id_1511
77. Prestwick0_000956
78. Prestwick1_000956
79. Prestwick2_000956
80. Prestwick3_000956
81. 6-methyl-delta(sup 4,6)-pregnadien-17-alpha-ol-3,20-dione Acetate
82. Schembl745
83. Bspbio_000952
84. Mls000759501
85. Mls001074091
86. Mls001424055
87. Par-100,2
88. Spbio_003101
89. Megestrol Acetate [mi]
90. Bpbio1_001048
91. Chebi:6723
92. Chembl1201139
93. Dtxsid9040683
94. Megestrol Acetate [vandf]
95. Megestrol Acetate [mart.]
96. Bcpp000168
97. Hms1570p14
98. Hms2051i20
99. Hms2090n04
100. Hms2097p14
101. Hms2235d16
102. Hms3714p14
103. Megestrol Acetate [usp-rs]
104. Megestrol Acetate [who-dd]
105. Megestrol Acetate [who-ip]
106. Nia
107. Nsc71423
108. Zinc4097467
109. Tox21_110469
110. Tox21_302360
111. Lmst02030118
112. Mfcd00056470
113. S1304
114. Akos015894927
115. Megestrol Acetate [green Book]
116. Tox21_110469_1
117. Bcp9000904
118. Ccg-100899
119. Cs-2065
120. Db00351
121. Megestrol Acetate [orange Book]
122. Nc00149
123. Megestrol Acetate [ep Monograph]
124. 17-acetoxy-6-methylpregna-4,20-dione
125. Megestrol Acetate [usp Monograph]
126. Ncgc00024196-03
127. Ncgc00024196-05
128. Ncgc00255456-01
129. Ac-24570
130. As-13384
131. Hy-13676
132. 17.alpha.-acetoxy-6-methyl-4,20-dione
133. Ab00490013
134. B1377
135. 17-hydroxy-6-methylpregna-4,20-dione Acetate
136. 17.alpha.-acetoxy-6-methylpregna-4,20-dione
137. C08151
138. D00952
139. D91560
140. Ab00383046-13
141. Ab00383046-14
142. Ab00383046-15
143. Ab00383046_16
144. Pregna-4,20-dione, 17-(acetyloxy)-6-methyl-
145. 595m335
146. A832354
147. Megestrol-17-acetate 1000 Microg/ml In Methanol
148. Sr-01000000258
149. Megestrol-17-acetate 100 Microg/ml In Acetonitrile
150. Pregna-4,20-dione, 17-hydroxy-6-methyl-, Acetate
151. Q-201346
152. Q6808975
153. Sr-01000000258-4
154. Brd-k19507340-001-03-1
155. Medroxyprogesterone Acetate Impurity G [who-ip]
156. Megestrol Acetate, Vetranal(tm), Analytical Standard
157. Megestrol-17-acetate 100 Microg/ml In Methanol/water
158. 17alpha-acetoxy-6-methyl-4,6-pregnadiene-3,20-dione
159. Megestrol Acetate, Analytical Standard, For Drug Analysis
160. Medroxyprogesterone Acetate Impurity G [ep Impurity]
161. Wln: L E5 B666 Ov Ku Mutj A1 E1 Fv1 Fov1 L1
162. 6-methyl-17.alpha.-hydroxy-.delta.(sup 6)-progesterone Acetate
163. Megestrol Acetate, European Pharmacopoeia (ep) Reference Standard
164. 6-methyl-3,20-dioxopregna-4,6-dien-17-yl Acetate [who-ip]
165. Megestrol Acetate, United States Pharmacopeia (usp) Reference Standard
166. Megesgtrol Acetate, Pharmaceutical Secondary Standard; Certified Reference Material
167. Megestrol Acetate For Peak Identification, European Pharmacopoeia (ep) Reference Standard
168. Megestrol Acetate For System Suitability, European Pharmacopoeia (ep) Reference Standard
| Molecular Weight | 384.5 g/mol |
|---|---|
| Molecular Formula | C24H32O4 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 384.23005950 g/mol |
| Monoisotopic Mass | 384.23005950 g/mol |
| Topological Polar Surface Area | 60.4 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 821 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Megace |
| PubMed Health | Megestrol Acetate (By mouth) |
| Drug Classes | Endocrine-Metabolic Agent |
| Drug Label | MEGACE (megestrol acetate, USP) Oral Suspension contains megestrol acetate, a synthetic derivative of the naturally occurring steroid hormone, progesterone. Megestrol acetate is a white, crystalline solid chemically designated as 17-(acetyloxy)-6... |
| Active Ingredient | Megestrol acetate |
| Dosage Form | Suspension |
| Route | Oral |
| Strength | 40mg/ml |
| Market Status | Prescription |
| Company | Bristol Myers Squibb |
| 2 of 6 | |
|---|---|
| Drug Name | Megace es |
| PubMed Health | Megestrol Acetate (By mouth) |
| Drug Classes | Endocrine-Metabolic Agent |
| Drug Label | Megace ES oral suspension contains megestrol acetate, a synthetic derivative of the naturally occurring steroid hormone, progesterone. Megestrol acetate is a white, crystalline solid chemically designated as 17-Hydroxy-6-methyl pregna-4,6-diene-3... |
| Active Ingredient | Megestrol acetate |
| Dosage Form | Suspension |
| Route | Oral |
| Strength | 125mg/ml |
| Market Status | Prescription |
| Company | Par Pharm |
| 3 of 6 | |
|---|---|
| Drug Name | Megestrol acetate |
| PubMed Health | Megestrol Acetate (By mouth) |
| Drug Classes | Endocrine-Metabolic Agent |
| Drug Label | Megestrol acetate is a synthetic, antineoplastic and progestational drug. Megestrol acetate is a white, crystalline solid chemically designated as 17(alpha)-(acetyloxy)-6-methylpregna-4,6-diene-3,20-dione. Solubility at 37C in water is 2 mcg per mL... |
| Active Ingredient | Megestrol acetate |
| Dosage Form | Tablet; Suspension |
| Route | Oral |
| Strength | 125mg/ml; 40mg/ml; 40mg; 20mg |
| Market Status | Prescription |
| Company | Twi Pharms; Wockhardt; Par Pharm; Roxane; Teva Pharms; Barr |
| 4 of 6 | |
|---|---|
| Drug Name | Megace |
| PubMed Health | Megestrol Acetate (By mouth) |
| Drug Classes | Endocrine-Metabolic Agent |
| Drug Label | MEGACE (megestrol acetate, USP) Oral Suspension contains megestrol acetate, a synthetic derivative of the naturally occurring steroid hormone, progesterone. Megestrol acetate is a white, crystalline solid chemically designated as 17-(acetyloxy)-6... |
| Active Ingredient | Megestrol acetate |
| Dosage Form | Suspension |
| Route | Oral |
| Strength | 40mg/ml |
| Market Status | Prescription |
| Company | Bristol Myers Squibb |
| 5 of 6 | |
|---|---|
| Drug Name | Megace es |
| PubMed Health | Megestrol Acetate (By mouth) |
| Drug Classes | Endocrine-Metabolic Agent |
| Drug Label | Megace ES oral suspension contains megestrol acetate, a synthetic derivative of the naturally occurring steroid hormone, progesterone. Megestrol acetate is a white, crystalline solid chemically designated as 17-Hydroxy-6-methyl pregna-4,6-diene-3... |
| Active Ingredient | Megestrol acetate |
| Dosage Form | Suspension |
| Route | Oral |
| Strength | 125mg/ml |
| Market Status | Prescription |
| Company | Par Pharm |
| 6 of 6 | |
|---|---|
| Drug Name | Megestrol acetate |
| PubMed Health | Megestrol Acetate (By mouth) |
| Drug Classes | Endocrine-Metabolic Agent |
| Drug Label | Megestrol acetate is a synthetic, antineoplastic and progestational drug. Megestrol acetate is a white, crystalline solid chemically designated as 17(alpha)-(acetyloxy)-6-methylpregna-4,6-diene-3,20-dione. Solubility at 37C in water is 2 mcg per mL... |
| Active Ingredient | Megestrol acetate |
| Dosage Form | Tablet; Suspension |
| Route | Oral |
| Strength | 125mg/ml; 40mg/ml; 40mg; 20mg |
| Market Status | Prescription |
| Company | Twi Pharms; Wockhardt; Par Pharm; Roxane; Teva Pharms; Barr |
For the treatment of anorexia, cachexia, or an unexplained, significant weight loss in patients with a diagnosis of acquired immunodeficiency syndrome (AIDS). Also used for the palliative management of recurrent, inoperable, or metastatic breast cancer, endometrial cancer, and prostate cancer in Canada and some other countries.
FDA Label
Megestrol is a synthetic progestin and has the same physiologic effects as natural progesterone. These effects include induction of secretory changes in the endometrium, increase in basal body temperature, pituitary inhibition, and production of withdrawal bleeding in the presence of estrogen. Mestrogel has slight glucocorticoid activity and very slight mineralocorticoid activity. This drug has no estrogenic, androgenic, or anabolic activity. The precise mechanism of megestrol’s antianorexic and anticachetic effects is unknown. Initially developed as a contraceptive, it was first evaluated in breast cancer treatment in 1967.
Antineoplastic Agents, Hormonal
Antineoplastic agents that are used to treat hormone-sensitive tumors. Hormone-sensitive tumors may be hormone-dependent, hormone-responsive, or both. A hormone-dependent tumor regresses on removal of the hormonal stimulus, by surgery or pharmacological block. Hormone-responsive tumors may regress when pharmacologic amounts of hormones are administered regardless of whether previous signs of hormone sensitivity were observed. The major hormone-responsive cancers include carcinomas of the breast, prostate, and endometrium; lymphomas; and certain leukemias. (From AMA Drug Evaluations Annual 1994, p2079) (See all compounds classified as Antineoplastic Agents, Hormonal.)
Appetite Stimulants
Agents that are used to stimulate appetite. These drugs are frequently used to treat anorexia associated with cancer and AIDS. (See all compounds classified as Appetite Stimulants.)
Absorption
Variable, but well absorbed orally.
Route of Elimination
The major route of drug elimination in humans is urine. Respiratory excretion as labeled carbon dioxide and fat storage may have accounted for at least part of the radioactivity not found in urine and feces.
Primarily hepatic. Megestrol metabolites which were identified in urine constituted 5% to 8% of the dose administered. Respiratory excretion as labeled carbon dioxide and fat storage may have accounted for at least part of the radioactivity not found in urine and feces. No active metabolites have been identified.
34 hours
The precise mechanism by which megestrol acetate produces effects in anorexia and cachexia is unknown at the present time, but its progestin antitumour activity may involve suppression of luteinizing hormone by inhibition of pituitary function. Studies also suggest that the megestrol's weight gain effect is related to its appetite-stimulant or metabolic effects rather than its glucocorticoid-like effects or the production of edema. It has also been suggested that megestrol may alter metabolic pathyways via interferences with the production or action of mediators such as cachectin, a hormone that inhibits adipocyte lipogenic enzymes.