


1. Menthae Piperitae Aetheroleum
2. Menthae Piperitae Oil
3. Oleum Menthae Piperitae
4. Ws 1340
5. Ws-1340
6. Ws1340
1. 8006-90-4
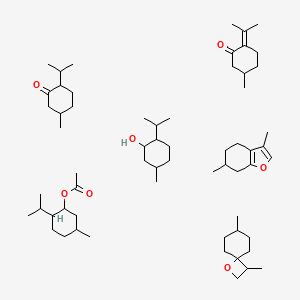
2. 3,7-dimethyl-1-oxaspiro[3.5]nonane;3,6-dimethyl-4,5,6,7-tetrahydro-1-benzofuran;5-methyl-2-propan-2-ylcyclohexan-1-ol;5-methyl-2-propan-2-ylcyclohexan-1-one;(5-methyl-2-propan-2-ylcyclohexyl) Acetate;5-methyl-2-propan-2-ylidenecyclohexan-1-one
| Molecular Weight | 965.5 g/mol |
|---|---|
| Molecular Formula | C62H108O7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 5 |
| Exact Mass | 964.80950578 g/mol |
| Monoisotopic Mass | 964.80950578 g/mol |
| Topological Polar Surface Area | 103 Ų |
| Heavy Atom Count | 69 |
| Formal Charge | 0 |
| Complexity | 953 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 11 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 6 |
Parasympatholytics; Antiemetics
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Peppermint oil is extensively used as a ... carminative, antiseptic, and local anesthetic in cold, cough, and other preparations (lozenges, syrups, ointments, tablets, etc.). Enteric coated peppermint oil capsules have been examined as a useful treatment for irritable bowel syndrome; enteric coating allows oil to reach colon in an unmetabolized state; treatment is contraindicated with meals ... and in achlorhydria. Peppermint oil has been recommended as an adjunct to colonoscopy; a diluted suspension of the oil is sprayed on the endoscope to reduce colonic spasm.
Leung, A.Y., Foster, S. Encyclopedia of Common Natural Ingredients Used in Food, Drugs, and Cosmetics. New York, NY. John Wiley & Sons, Inc. 1996., p. 370
TRADITIONAL MEDICINE: Peppermint, spearmint, and their oils reportedly used in both western and eastern cultures as aromatic, stomachic, stimulant, antiseptic, local anesthetic, and antispasmodic in treating indigestion, nausea, sore throat, diarrhea, colds, headaches, toothaches, and cramps. ... /has/ been reported used in cancers.
Leung, A.Y., Foster, S. Encyclopedia of Common Natural Ingredients Used in Food, Drugs, and Cosmetics. New York, NY. John Wiley & Sons, Inc. 1996., p. 370
MEDICATION (VET): CARMINATIVE
The Merck Index. 9th ed. Rahway, New Jersey: Merck & Co., Inc., 1976.
Indicated for the over-the-counter use for: -the symptomatic relief of minor spasms of the gastrointestinal tract, flatulence and abdominal pain, especially in patients with irritable bowel syndrome. -the temporary relief of itching associated with insect bites, eczema, minor burn, sunburn, minor skin irritations, minor cuts, scrapes, atopic dermatitis and other skin disorders. -the temporary symptomatic relief of mild joint and muscle pain as a local topical analgesic. -the temporary relief of tension-type headache.
Peppermint oil induces a dose-related antispasmodic effects on the gastrointestinal smooth muscles. A meta-analysis study and additional clinical studies of patients with IBS demonstrated that the treatment with peppermint oil improves abdominal symptoms compared to the placebo group, resulting in reduced severity of abdominal pain, decreased abdominal distension, reduced stool frequency, and reduced flatulence. The use of enteric-coated peppermint oil was shown to be effective in reducing gastrointestinal symptoms of non-ulcer dyspepsia. In rats, peppermint oil promoted a time-dependent choleretic effect in increasing bile production and biliary output. In randomized controlled trials, topical application of peppermint oil was associated with a significant analgesic effect and a reduction in headache intensity compared to placebo. In a study of C57BL/6 mice, topical application of peppermint oil for 4 weeks was associated with a prominent hair growth effects; a significant increase in dermal thickness, follicle number, and follicle depth.
Antiemetics
Drugs used to prevent NAUSEA or VOMITING. (See all compounds classified as Antiemetics.)
Parasympatholytics
Agents that inhibit the actions of the parasympathetic nervous system. The major group of drugs used therapeutically for this purpose is the MUSCARINIC ANTAGONISTS. (See all compounds classified as Parasympatholytics.)
Absorption
After oral administration, peppermint is rapidly absorbed. Menthol is highly fat-soluble therefore rapidly absorbed from the proximal gut.
Route of Elimination
Peppermint oil is eliminated mainly via the bile following oral administration, with glucuronide and sulphate metabolites predominant. The metabolites, mainly menthol glucuronide and mono- or di-hydroxylated menthol derivatives, may also undergo approximately equal renal and fecal excretion. Renal recovery of total menthol within 24 hours was dose-dependent whereas the recovery in bile was substantially higher over 8 hours.
Volume of Distribution
No pharmacokinetic data available.
Clearance
No pharmacokinetic data available.
The predominant biliary metabolite of peppermint oil is menthol glucuronide, which undergoes enterohepatic circulation. The urinary metabolites are products of hydroxylation at the C-7 methyl group at C-8 and C-9 of the isopropyl moiety, forming a series of mono- and dihydroxymenthols and carboxylic acids, some of which are excreted in part as glucuronic acid conjugates.
No pharmacokinetic data available.
Dose-dependent antispasmodic effect of peppermint oil is largely mediated by its menthol constituent. It is proposed that peppermint oil relaxes gastrointestinal smooth muscle and attenuates contractile responses by reducing the influx of extracellular calcium ions. In rabbit jejunum smooth muscle cells investigated via whole cell clamp configuration technique, peppermint oil was shown to inhibit the potential-dependent calcium currents in a concentration-dependent manner. Both a reduction in peak current amplitude and an increase in the rate of current decay were observed, indicating that the pharmacological activity peppermint oil resembles that of dihydropyridine calcium antagonists. In a rat small intestine study, peppermint oil in the intestinal lumen inhibited enterocyte glucose uptake via a direct action on the brush border membrane and inhibited intestinal secretion. There is also evidence that menthol is an antagonist of L-type Ca2+ channels via interacting with dihydropyridine binding sites and blocks the currents of low-voltage-activated calcium channels. Peppermint oil may facilitate hair growth by promoting the conservation of vascularization of hair dermal papilla, which may contribute to the induction of early anagen stage of active growth phase of hair follicles.