

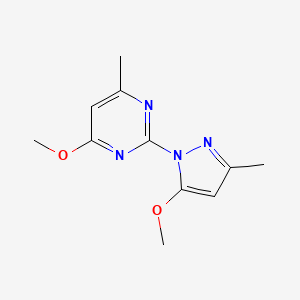

1. Mepirizole
2. Methopyrimazole
1. Mepirizole
2. 18694-40-1
3. Mebron
4. Methopyrimazole
5. 4-methoxy-2-(5-methoxy-3-methyl-1h-pyrazol-1-yl)-6-methylpyrimidine
6. Mepirizol
7. Da-398
8. Epirizolum [inn-latin]
9. 4-methoxy-2-(5-methoxy-3-methylpyrazol-1-yl)-6-methylpyrimidine
10. Pyrimidine, 4-methoxy-2-(5-methoxy-3-methyl-1h-pyrazol-1-yl)-6-methyl-
11. Mls000028844
12. 3b46o2fh8i
13. 1-(4-methoxy-6-methyl-2-pyrimidinyl)-3-methyl-5-methoxypyrazole
14. 2-(3-methoxy-5-methylpyrazol-2-yl)-4-methoxy-6-methylpyrimidine
15. 2-(3-methyl-5-methoxy-1-pyrazolyl)-4-methoxy-6-methylpyrimidine
16. Ncgc00016737-01
17. Smr000058715
18. 4-methoxy-2-(5-methoxy-3-methylpyrazol-1-yl)-6-methylpyrimidin
19. Cas-18694-40-1
20. Dsstox_cid_25422
21. Dsstox_rid_80869
22. Dsstox_gsid_45422
23. Mepirizol [german]
24. Epirizolum
25. Epirizol
26. Epirizol [inn-spanish]
27. Pyrimidine,4-methoxy-2-(5-methoxy-3-methyl-1h-pyrazol-1-yl)-6-methyl-
28. Epirizole [usan:inn:jan]
29. Einecs 242-507-1
30. Brn 0751084
31. Unii-3b46o2fh8i
32. Prestwick_201
33. Mebron (tn)
34. Epirizole [inn]
35. Epirizole [jan]
36. Opera_id_598
37. Epirizole [mi]
38. Epirizole [usan]
39. Prestwick0_000032
40. Prestwick1_000032
41. Prestwick2_000032
42. Prestwick3_000032
43. Epirizole [mart.]
44. 4-methoxy-2-(5-methoxy-3-methylpyrazol-1-yl)-6-methylpyrimidin [german]
45. Epirizole (jp17/usan)
46. Epirizole [who-dd]
47. Schembl23765
48. Bspbio_000123
49. 5-25-18-00012 (beilstein Handbook Reference)
50. Mls001148256
51. Mls001424179
52. Spbio_002044
53. Mepirizole, Analytical Standard
54. Bpbio1_000137
55. Chembl1411693
56. Dtxsid4045422
57. Chebi:31545
58. Zinc57412
59. Hms1568g05
60. Hms2052a07
61. Hms2095g05
62. Hms2232e23
63. Hms3370d19
64. Hms3394a07
65. Hms3712g05
66. Tox21_110584
67. Pyrimidine, 4-methoxy-2-(5-methoxy-3-methylpyrazol-1-yl)-6-methyl-
68. Akos016000700
69. Tox21_110584_1
70. Ccg-101087
71. Db08991
72. Nc00337
73. Ncgc00016737-02
74. Ncgc00016737-04
75. Hy-103595
76. Cs-0022015
77. Ft-0637738
78. Mls000028844-02
79. D01394
80. 694m401
81. Sr-01000721915
82. J-012020
83. Q5383219
84. Sr-01000721915-3
85. Brd-k39339537-001-03-8
86. 2-(3-methyl-5-methoxy-1-pyrazolyl)-4-methoxy-6-methyl-pyrimidine
| Molecular Weight | 234.25 g/mol |
|---|---|
| Molecular Formula | C11H14N4O2 |
| XLogP3 | 1.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 3 |
| Exact Mass | 234.11167570 g/mol |
| Monoisotopic Mass | 234.11167570 g/mol |
| Topological Polar Surface Area | 62.1 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 254 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
