


1. 10364-s
2. 2-alpha,3 Alpha-epithio-5 Alpha-androstan-17 Beta-yl-1-methoxycyclopentyl Ether
1. 21362-69-6
2. Thioderon
3. O00404969k
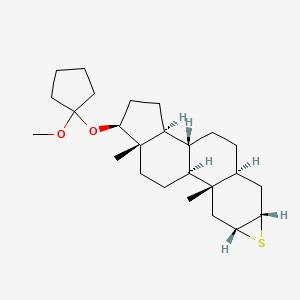
4. (1s,3as,3br,5as,6as,7ar,8as,8bs,10as)-1-((1-methoxycyclopentyl)oxy)-8a,10a-dimethylhexadecahydro-1h-cyclopenta[7,8]phenanthro[2,3-b]thiirene
5. Mepitiostano
6. Mepitiostanum
7. Thioderon (tn)
8. Mepitiostane [inn:jan]
9. Mepitiostane [mi]
10. Mepitiostane [inn]
11. Mepitiostane [jan]
12. Mepitiostanum [inn-latin]
13. Mepitiostane (jp17/inn)
14. Mepitiostano [inn-spanish]
15. Schembl13123
16. Mepitiostane [mart.]
17. Mepitiostane [who-dd]
18. Ccris 2779
19. Chembl2104860
20. Chebi:31818
21. Dtxsid001016882
22. Unii-o00404969k
23. Zinc4216860
24. Akos015904663
25. Ncgc00522025-01
26. 10364s
27. D01602
28. S 10364
29. Q6817819
30. 2-alpha,3-alpha-epithio-17-beta-yl 1-methoxycyclopentyl Ether
31. 5-alpha-androstane, 2-alpha,3-alpha-epithio-17-beta-(1-methoxycyclopentyloxy)-
32. Androstane, 2,3-epithio-17-((1-methoxycyclopentyl)oxy)-, (2alpha,3alpha,5alpha,17beta)-
33. Cyclopentanone 2alpha,3alpha-epithio-5alpha-androstan-17beta-yl Methyl Acetal
34. Cyclopentanone, 2-alpha, 3-alpha-epithio-5-alpha-androstan-17-beta-yl Methyl Acetal
35. (1s,2s,4r,6s,8s,11r,12s,15s,16s)-15-(1-methoxycyclopentyl)oxy-2,16-dimethyl-5-thiapentacyclo[9.7.0.02,8.04,6.012,16]octadecane
36. Androstane, 2,3-epithio-17-((1-methoxycyclopentyl)oxy)-, (2-alpha,3-alpha,5-alpha,17-beta)
37. Cyclopentanone 2.alpha.,3.alpha.-epithio-5.alpha.-androstan-17.beta.-yl Methyl Acetal
| Molecular Weight | 404.7 g/mol |
|---|---|
| Molecular Formula | C25H40O2S |
| XLogP3 | 6.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 404.27490169 g/mol |
| Monoisotopic Mass | 404.27490169 g/mol |
| Topological Polar Surface Area | 43.8 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 630 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anabolic Agents
These compounds stimulate anabolism and inhibit catabolism. They stimulate the development of muscle mass, strength, and power. (See all compounds classified as Anabolic Agents.)
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Estrogen Antagonists
Compounds which inhibit or antagonize the action or biosynthesis of estrogenic compounds. (See all compounds classified as Estrogen Antagonists.)