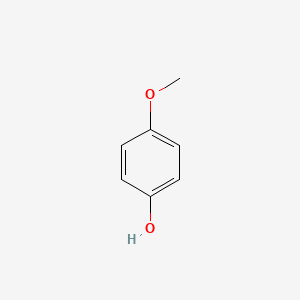

1. 4-hydroxyanisole
2. 4-hydroxyanisole, Potassium Salt
3. 4-hydroxyanisole, Sodium Salt
4. Hydroquinone Methyl Ether
5. Hydroquinone Monomethyl Ether
6. Leucodinine B
7. Mequinol
8. P-hydroxyanisole
9. Para-methoxyphenol
1. Mequinol
2. 150-76-5
3. 4-hydroxyanisole
4. P-hydroxyanisole
5. P-methoxyphenol
6. Phenol, 4-methoxy-
7. Hydroquinone Monomethyl Ether
8. Leucobasal
9. Mehq
10. Leucodine B
11. Mechinolum
12. P-guaiacol
13. Hydroquinone Methyl Ether
14. Novo-dermoquinona
15. Hqmme
16. P-hydroxymethoxybenzene
17. Para-methoxyphenol
18. 1-hydroxy-4-methoxybenzene
19. Monomethyl Ether Hydroquinone
20. 4-methoxy-phenol
21. Pmf (antioxidant)
22. Phenol, P-methoxy-
23. Usaf An-7
24. Mono Methyl Ether Hydroquinone
25. Nsc 4960
26. Mfcd00002332
27. Bms 181158
28. Bms-181158
29. Nsc-4960
30. 6ht8u7k3am
31. Dtxsid4020828
32. Chebi:69441
33. Nsc4960
34. Mequinol (inn)
35. Ncgc00091390-02
36. Mequinol [inn]
37. Dsstox_cid_828
38. Dsstox_rid_75814
39. Dsstox_gsid_20828
40. Mechinolo [dcit]
41. Mequinolum
42. Mechinolo
43. Mequinolum [inn-latin]
44. Cas-150-76-5
45. Ccris 5531
46. Hsdb 4258
47. Einecs 205-769-8
48. Unii-6ht8u7k3am
49. Mequinol [usan:inn:dcf]
50. 4methoxyphenol
51. Paramethoxyphenol
52. Ai3-00841
53. P- Methoxyphenol
54. P-methoxy Phenol
55. P-methoxy-phenol
56. 4-methoxy Phenol
57. Eastman Hqmme
58. 4-(methoxy)phenol
59. 4ha
60. 4ks
61. Para- Hydroxyanisole
62. 4-(methyloxy)phenol
63. Hqme
64. Mequinol [hsdb]
65. Mequinol [usan]
66. Mequinol (usan/inn)
67. Mequinol, Inn, Usan
68. Mequinol [vandf]
69. Phenol,4-methoxy
70. Hydroxyquinone Methyl Ether
71. Mequinol [mart.]
72. Hydroquinone Monomethylether
73. Chembl544
74. Mequinol [who-dd]
75. Ec 205-769-8
76. Ncimech_000709
77. Wln: Qr Do1
78. Schembl21009
79. Hydroquinone Mono Methyl Ether
80. Mls002454409
81. Mequinol [orange Book]
82. Gtpl6827
83. Zinc1684
84. P-hydroxyanisole [inci]
85. Solage Component Mequinol
86. Schembl12015251
87. Bdbm36295
88. Hms2270f04
89. Hms3264p13
90. Hms3652o08
91. Pharmakon1600-00212037
92. Mequinol Component Of Solage
93. 4-methoxyphenol, Analytical Standard
94. Tox21_111125
95. Tox21_202367
96. Tox21_302876
97. Ccg-35855
98. Nsc760357
99. Stl199145
100. Akos000119852
101. Tox21_111125_1
102. Ac-3292
103. Am10685
104. Cs-w019963
105. Db09516
106. Nsc-760357
107. Ps-3375
108. Sb40551
109. 4-methoxyphenol, Reagentplus(r), 99%
110. Ncgc00091390-01
111. Ncgc00091390-03
112. Ncgc00091390-04
113. Ncgc00256552-01
114. Ncgc00259916-01
115. Bp-23487
116. Hqmme; Hydroxyquinone Methyl Ether
117. Hy-30270
118. Nci60_004190
119. Smr001252253
120. Db-003965
121. B1968
122. Ft-0618865
123. M0123
124. S4077
125. Sw219760-1
126. 4-methoxyphenol, Purum, >=98.0% (hplc)
127. 4-methoxyphenol, Saj First Grade, >=97.0%
128. D04926
129. P17835
130. Ab00641905_06
131. Ab00641905_07
132. A809071
133. Sr-01000865565
134. Q-200491
135. Q2862455
136. Sr-01000865565-2
137. Brd-k45216060-001-06-8
138. F9995-1658
139. Z1262246146
140. 4-methoxybenzyl S-(4,6-dimethylpyrimidin-2-yl)thiocarbonate
| Molecular Weight | 124.14 g/mol |
|---|---|
| Molecular Formula | C7H8O2 |
| XLogP3 | 1.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 124.052429494 g/mol |
| Monoisotopic Mass | 124.052429494 g/mol |
| Topological Polar Surface Area | 29.5 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 75 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antineoplastic Agents; Antioxidants
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Dyschromias, in particular hyperpigmentation, are major issues of concern for people of color. Pigmentary disorders such as melasma and postinflammatory hyperpigmentation (PIH) can cause psychological and emotional distress and can pose a negative impact on a person's health-related quality of life. The precise etiology of these conditions is unknown. Therapies for melasma and PIH target various points during the cycle of melanin production and degradation. Therapies for these conditions include topical agents and resurfacing procedures. Hydroquinone remains the gold standard of topical agents. Other efficacious agents include kojic acid, azelaic acid, mequinol, and retinoids. Cosmeceutical agents include licorice, arbutin, soy, N-acetyl glucosamine, and niacinamide. Resurfacing procedures that have been used to treat melasma and PIH include chemical peels, microdermabrasion, lasers, and intense pulsed light. These procedures are best used in combination with topical bleaching agents. Given the propensity of darker skin to hyperpigment, resurfacing procedures should be used with care and caution. Maximal results are best achieved with repetitive, superficial, resurfacing modalities. In addition, ultraviolet protective measures such as broad-spectrum sunscreens are fundamental to the successful management of these conditions.
PMID:19608057 Grimes PE; Semin Cutan Med Surg 28(2):77-85 (2009).
Postinflammatory hyperpigmentation (PIH) is a reactive hypermelanosis and sequela of a variety of inflammatory skin conditions. PIH can have a negative impact on a patient's quality of life, particularly for darker-skinned patients. Studies show that dyschromias, including PIH, are one of the most common presenting complaints of darker-skinned racial ethnic groups when visiting a dermatologist. This is likely due to an increased production or deposition of melanin into the epidermis or dermis by labile melanocytes. A variety of endogenous or exogenous inflammatory conditions can culminate in PIH and typically most epidermal lesions will appear tan, brown, or dark brown while dermal hypermelanosis has a blue-gray discoloration. Depigmenting agents target different steps in the production of melanin, most commonly inhibiting tyrosinase. These agents include hydroquinone, azelaic acid, kojic acid, arbutin, and certain licorice (glycyrrhiza) extracts. Other agents include retinoids, mequinol, ascorbic acid (vitamin C), niacinamide, N-acetyl glucosamine, and soy, and these products depigment by different mechanisms. Certain procedures can also be effective in the treatment of PIH including chemical peeling and laser therapy. It is important to note that these same therapeutic modalities may also play a role in causing PIH. Lastly, those lesions that are not amenable to medical or surgical therapy may experience some improvement with cosmetic camouflage.
PMID:21348540 Callender VD et al; Am J Clin Dermatol 12(2):87-99 (2011).
Neither the safety nor effectiveness of Solage Solution for the prevention or treatment of melasma or postinflammatory hyperpigmentation has been established. /Included in US product label/ /Solage/
US Natl Inst Health; DailyMed. Current Medication Information. Solage (03/2007). Available from, as of October 15, 2008: https://dailymed.nlm.nih.gov/dailymed/about.cfm
For more Therapeutic Uses (Complete) data for 4-METHOXYPHENOL (9 total), please visit the HSDB record page.
Known hypersensitivity to mequinol or tretinoin or any ingredient in the formulation. /Solage/
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015
Mequinol and tretinoin should be used with extreme caution in patients with eczema, since tretinoin may produce severe irritation of eczematous skin. In addition, mequinol and tretinoin is a dermal irritant and the risk of long-term sequelae from continued skin irritation for longer than 52 weeks is not known. Irritation of the skin induced by the drug may result in increased reactivity to environmental (eg, wind and cold exposure) stimuli. If local irritation becomes severe, temporary or permanent discontinuance of the drug or dosage reduction should be considered. /Solage/
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015
Safety and efficacy of mequinol and tretinoin have not been established in patients with moderately or heavily pigmented skin. /Solage/
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015
Patients with a personal or family history of vitiligo may experience enhanced response to mequinol and tretinoin topical solution. During clinical trials, one patient with a family history of vitiligo experienced hypopigmentation in areas not treated with the drug and some areas continued to worsen for at least 1 month after discontinuance of therapy; hypopigmentation became mild after 6 weeks and resolved in some, but not all, lesions by day 106 post-treatment. /Solage/
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015
For more Drug Warnings (Complete) data for 4-METHOXYPHENOL (13 total), please visit the HSDB record page.
Mequinol is currently primarily available only as an active ingredient in combination products combined with tretinoin that are indicated for the treatment of solar lentigines and related hyperpigmented lesions resulting from chronic sun exposure.
FDA Label
Mequinol is in fact considered a melanocytotoxic chemical which when oxidized in melanocytes results in the formation of toxic entities like quinones. Such cytotoxic compounds subsequently have the potential to damage and destroy pigment cells, therefore causing skin depigmentation. In response, skin cells are naturally capable of protecting themselves against such cytotoxic agents with the help of endogenous intracellular glutathione and the detoxification action of glutathione S-transferase on the cytotoxic compounds. Regardless, it is consequently by way of this seemingly negative and damaging pharmacodynamic profile by which the mechanism of action of mequinol is sometimes described.
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
D - Dermatologicals
D11 - Other dermatological preparations
D11A - Other dermatological preparations
D11AX - Other dermatologicals
D11AX06 - Mequinol
Absorption
The systemic exposure to mequinol was assessed in eight healthy subjects following two weeks of twice-daily topical treatment of a tretinoin and mequinol combination product. About dose of the product corresponding to about 37.3 ug/cm^2 of mequinol was applied to the subjects' backs. The mean Cmax for mequinol was 9.92 ng/mL (range between 4.22 and 23.62 ng/mL) and the Tmax was 2 hours (range between 1 to 2 hours). The safety of mequinol in this combination formulation is supported by the low systemic exposures of the agent in the subjects.
Route of Elimination
Mequinol is predominantly renally eliminated as its metabolites.
Volume of Distribution
The volume of distribution is one that suggests mequinol is distributed throughout the total body water, and intracellular concentrations are not expected to vary greatly from gross measurements.
Clearance
Readily accessible data regarding the clearance of mequinol is not available. The use of mequinol containing products is typically indicated for topical use.
Solage is a combination product composed of 2% mequinol (4-hydroxyanisole) and 0.01% tretinoin (all-trans-retinoic acid) in an ethanolic solution ... The purpose of this study was to evaluate the extent of percutaneous absorption of [(3)H]tretinoin and to estimate the systemic exposure to mequinol from this combination product when topically applied to the backs of healthy subjects. Eight subjects received bid /twice per day/ topical applications of nonradiolabelled 2% mequinol/0.01% tretinoin solution on a 400 sq cm area of the back for 14 days. The subjects then received a single topical application of 2% mequinol/0.01% ((3)H)tretinoin solution. After 12 hr, the radiolabelled dose was removed and bid /twice per day/ treatment with nonradiolabelled 2% mequinol/0.01% tretinoin solution was continued for 7 days. Plasma, urine and faecal samples were analysed for total radioactivity and plasma was analysed for both mequinol and tretinoin by GC/MS procedure. Mean percutaneous absorption of [(3)H]tretinoin based on the cumulative recoveries of radioactivity in the urine and faeces was about 4.5% (median 2.18%). Tretinoin concentrations in plasma did not increase above endogenous levels. This was consistent with the concentrations of radioactivity in plasma, which showed an average Cmax of 91 pg-eq/mL (median 26 ng/mL). Average Cmax and AUC(0-12 hr) values for mequinol were 10 ng/mL and 33 ng h/mL, respectively. Based on the results of this study, systemic toxicity from topical application of tretinoin in this formulation is unlikely, because percutaneous absorption of tretinoin is minimal and because endogenous levels of tretinoin are not increased following bid /twice per day/ dosing with this combination formulation. The safety of mequinol in this combination formulation is supported by the low systemic exposures of the subjects in this study compared with the systemic exposures at the highest doses in the dermal toxicity studies in mice (16.6-fold) and rats (34.6-fold).
PMID:10701701 Everett DW, Franz TJ, Chando TJ, Gale PJ, Lehman PA, Schwarzel EH, Parab PV, D'Arienzo CJ, Kripalani KJ Biopharm; Drug Dispos 20(6):301-8 (1999)
In one study in healthy individuals, following topical application of 0.8 mL of the combination preparation containing mequinol 2% and tretinoin 0.01% on a 400-sq cm back skin area twice daily for 14 days, approximately 4.5% of a radiolabeled dose was recovered as tretinoin in urine and feces. In this study, plasma tretinoin concentrations did not increase above endogenous plasma concentrations and average peak plasma mequinol concentrations were about 10 ng/mL. /Solage/
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015
There is also indication ... that the material was absorbed in toxic amounts when in solution, especially through abraded skin.
Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 2530
Urine samples from melanoma patients treated with mequinol were analyzed and various mequinol metabolites were identified, including 3,4-dihydroxyanisole, the two o-methyl derivatives 3-hydroxy-4-methoxyanisole and 4-hydroxy-3-methoxyanisole, and even hydroquinone which may have originated at least partly from mequinol. All these identified metabolites were excreted predominantly as sulphates and glucuronides - only a small portion of the substances were present in urine in an unconjugated form. Ultimately, the 3,4-dihydroxyanisole is considered the most important metabolite of mequinol.
A tyrosinase-directed therapeutic approach for treating malignant melanoma uses depigmenting phenolic prodrugs such as 4-hydroxyanisole (4-HA) for oxidation by melanoma tyrosinase to form cytotoxic o-quinones. However, in a recent clinical trial, both renal and hepatic toxicity were reported as side effects of 4-HA therapy. In the following, 4-HA (200 mg/kg i.p.) administered to mice caused a 7-fold increase in plasma transaminase toxicity, an indication of liver toxicity. Furthermore, 4-HA induced-cytotoxicity toward isolated hepatocytes was preceded by glutathione (GSH) depletion, which was prevented by cytochrome p450 inhibitors that also partly prevented cytotoxicity. The 4-HA metabolite formed by NADPH/microsomes and GSH was identified as a hydroquinone mono-glutathione conjugate. GSH-depleted hepatocytes were much more prone to cytotoxicity induced by 4-HA or its reactive metabolite hydroquinone (HQ). Dicumarol (an NAD(P)H/quinone oxidoreductase inhibitor) also potentiated 4-HA- or HQ-induced toxicity whereas sorbitol, an NADH-generating nutrient, prevented the cytotoxicity. Ethylenediamine (an o-quinone trap) did not prevent 4-HA-induced cytotoxicity, which suggests that the cytotoxicity was not caused by o-quinone as a result of 4-HA ring hydroxylation. Deferoxamine and the antioxidant pyrogallol/4-hydroxy-2,2,6,6-tetramethylpiperidene-1-oxyl (TEMPOL) did not prevent 4-HA-induced cytotoxicity, therefore excluding oxidative stress as a cytotoxic mechanism for 4-HA. A negligible amount of formaldehyde was formed when 4-HA was incubated with rat microsomal/NADPH. These results suggest that the 4-HA cytotoxic mechanism involves alkylation of cellular proteins by 4-HA epoxide or p-quinone rather than involving oxidative stress.
PMID:12228181 Moridani MY, Cheon SS, Khan S, O'Brien PJ; Drug Metab Dispos. 30(10):1063-9 (2002)
Many of the well-known depigmenting agents such as hydroquinone and 4-hydroxyanisole are, in fact, melanocytotoxic chemicals which are oxidized in melanocytes to produce highly toxic compounds such as quinones. These cytotoxic compounds are responsible for the destruction of pigment cells, which results in skin depigmentation. However, cells are capable of protecting themselves against cytotoxic agents by intracellular glutathione (GSH). This protection takes place under the enzymatic action of the detoxification enzyme glutathione S-transferase (GST), which is responsible for the conjugation of toxic species to GSH. The depigmenting effect of hydroquinone is shown to be potentiated by buthionine sulfoximine (BSO) and cystamine as the result of the reduction of intracellular levels of GSH by these two agents. Additionally, BSO and cystamine are shown to inhibit the activity of GST. The combination of all-trans-retinoic acid (tretinoin, TRA) with hydroquinone or 4-hydroxyanisole is also known to produce synergetic skin depigmentation. TRA serves as a potent inhibitor of mammalian GSTs and is known to make cells more susceptible to the cytotoxic effect of chemicals by inhibiting the activity of this enzyme. This agent is also shown to reduce the level of intracellular GSH in certain cells. We have proposed that the mechanism of action of TRA to synergistically enhance the melanocytotoxic effect of chemicals involves the inhibition of GST and the impairment of glutathione-dependent cytoprotection against melanocytotoxic agents.
PMID:12771467 Kasraee B, Handjani F, Aslani FS; Dermatology 206(4):289-91 (2003)
Yields 1,4-dimethoxybenzene in guinea pig, rat, rabbit, mouse. Yields 4-methoxycatechol, p-methoxyphenyl-beta-d-glucuronide, & p-methoxyphenyl sulfate in rabbit. /From Table/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. M-15
4-Methoxyphenol has known human metabolites that include (2S,3S,4S,5R)-3,4,5-Trihydroxy-6-(4-methoxyphenoxy)oxane-2-carboxylic acid.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Mequinol demonstrated an elimination half-life of 30 to 90 minutes following intravenous infusion of 5 or 10 grams/m^2 over 3 to 5 hours in melanoma patients; similar values were reported after intra-arterial infusion.
Solar lentigines and related hyperpigmented lesions are localized, pigmented, macular lesions of the skin, usually on the areas of the body which have been chronically exposed to sunlight. These lesions are characterized by increased numbers of active melanocytes and increased melanin production. Although the mechanism of action of mequinol is not fully elucidated, when employed as an active ingredient in combination with other agents like tretinoin in skin depigmentation products, a synergy between a number of potential mechanisms is proposed. Firstly, mequinol is in fact considered a melanocytotoxic chemical which when oxidized in melanocytes results in the formation of toxic compounds like quinones. Such cytotoxic agents are subsequently capable of damaging and destroying pigment cells, which results in skin depigmentation of solar lentigines or other related hyperpigmented lesions. Nevertheless, skin cells are naturally capable of protecting themselves against such cytotoxic entities by endogenous intracellular glutathione (GSH). This protection is elicited through the enzymatic action of glutathione S-transferase (GST), which is responsible for the conjugation of agents toxic to glutathione. Conversely, tretinoin has been observed to serve as a potent inhibitor of mammalian GSTs and to be capable of reducing the level of intracellular GSH in various cells. As a result, the combination of mequinol with tretinoin seemingly allows for a synergistic enhancement of a melanocytotoxic effect that involves the inhibition and impairment of GSH and GST cytoprotection. Secondly, even though mequinol is a substrate for the enzyme tyrosinase and therefore acts as a competitive inhibitor of the formation of melanin precursors by way of tyrosinase facilitated reactions, the clinical significance of this action is unknown.
We examined changes in the levels of chaperone proteins to evaluate the toxic effects of environmental chemicals in human cells in vitro. Some chaperones are up-regulated by estrogenic chemicals, but the effect is not necessarily dependent on the receptor. Thus we also investigated whether a chemical-induced change in chaperone protein expression is human estrogen receptor (hER)-dependent or not, using cultured human cell lines transfected with hERalpha cDNA or an empty vector. In the hERalpha-expressed cells, the protein levels of the heat shock protein 27 (HSP27), the glucose-regulated protein 78 (GRP78/BiP), and GRP94 increased after exposure to beta-estradiol (E(2)) (from 10(-9)M to 10(-6)M) and bisphenol A (BPA) (from 10(-6)M to 10(-5)M). On the other hand, the increase was not observed in the cells without hERalpha expression. These results suggest that the E(2)- and BPA-induced increase in the protein levels were hERalpha dependent. We next examined the effect of four phenolic chemicals similar in structure to BPA, and found that among them, 4-methoxyphenol (from 10(-6)M to 10(-5)M) increased the levels of the chaperone proteins with hERalpha dependency. Thus the human cultured cells would be suitable for evaluating whether an increase in chaperone proteins occurs upon exposure to environmental chemicals and whether the effect is ER-dependent.
PMID:19269315 Kita K et al; Toxicol In Vitro. 23(4):728-35 (2009).
Many of the well-known depigmenting agents such as hydroquinone and 4-hydroxyanisole are, in fact, melanocytotoxic chemicals which are oxidized in melanocytes to produce highly toxic compounds such as quinones. These cytotoxic compounds are responsible for the destruction of pigment cells, which results in skin depigmentation. However, cells are capable of protecting themselves against cytotoxic agents by intracellular glutathione (GSH). This protection takes place under the enzymatic action of the detoxification enzyme glutathione S-transferase (GST), which is responsible for the conjugation of toxic species to GSH. The depigmenting effect of hydroquinone is shown to be potentiated by buthionine sulfoximine (BSO) and cystamine as the result of the reduction of intracellular levels of GSH by these two agents. Additionally, BSO and cystamine are shown to inhibit the activity of GST. The combination of all-trans-retinoic acid (tretinoin, TRA) with hydroquinone or 4-hydroxyanisole is also known to produce synergetic skin depigmentation. TRA serves as a potent inhibitor of mammalian GSTs and is known to make cells more susceptible to the cytotoxic effect of chemicals by inhibiting the activity of this enzyme. This agent is also shown to reduce the level of intracellular GSH in certain cells. We have proposed that the mechanism of action of TRA to synergistically enhance the melanocytotoxic effect of chemicals involves the inhibition of GST and the impairment of glutathione-dependent cytoprotection against melanocytotoxic agents.
PMID:12771467 Kasraee B, Handjani F, Aslani FS; Dermatology 206(4):289-91 (2003)
Mequinol, the monomethyl ether of hydroquinone, is a substrate for the enzyme tyrosinase and acts as a competitive inhibitor of the formation of melanin precursors. The precise mechanism of action of mequinol is not known. /Solage/
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015