

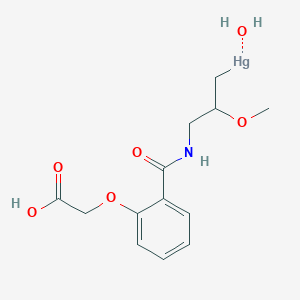

1. Acid, Mersalyl
2. Mercuramide
3. Mercusal
4. Mersalin
5. Mersalyl
6. Salyrgan
1. Mersalyl
2. 486-67-9
3. Ncgc00181320-01
4. [3-[[2-(carboxymethoxy)benzoyl]amino]-2-methoxypropyl]mercury;hydrate
5. Mersal
6. (3-(2-(carboxymethoxy)benzamido)-2-methoxypropyl)(hydroxy)mercury
7. {3-[2-(carboxymethoxy)benzamido]-2-methoxypropyl}(hydroxy)mercury
8. Dsstox_cid_26902
9. Dsstox_rid_82001
10. Dsstox_gsid_46902
11. Schembl993248
12. Chebi:6771
13. Gtpl5331
14. Dtxsid101334269
15. Mersalyl Acid, Analytical Standard
16. Tox21_112788
17. Db09338
18. Cas-486-67-9
19. Q424871
20. 879642-25-8
21. Mercurate(1-), [3-[[2-(carboxylatomethoxy)benzoyl]amino]-2-methoxypropyl]hydroxy-, Hydrogen
| Molecular Weight | 484.88 g/mol |
|---|---|
| Molecular Formula | C13H18HgNO6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 9 |
| Exact Mass | 486.084056 g/mol |
| Monoisotopic Mass | 486.084056 g/mol |
| Topological Polar Surface Area | 85.9 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 328 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Elevated blood pressure, edema.
Mersalyl acid is an organomercuric compound. It is used as a diuretic. Mercury is a heavy, silvery d-block metal and one of six elements that are liquid at or near room temperature and pressure. It is a naturally occuring substance, and combines with other elements such as chlorine, sulfur, or oxygen to form inorganic mercury compounds (salts). Mercury also combines with carbon to make organic mercury compounds.
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
C - Cardiovascular system
C03 - Diuretics
C03B - Low-ceiling diuretics, excl. thiazides
C03BC - Mercurial diuretics
C03BC01 - Mersalyl
Route of Elimination
Organic mercury is metabolized into inorganic mercury, which is eventually excreted in the urine and feces.
Clearance
The hepatobiliary excretion of mersayl was studied in the isolated perfused rat liver and in isolated rat liver plasma membrane vesicles. In the isolated perfused liver, mersalyl was found to be immediately absorbed by the perfusion medium and concentratively excreted into bile. Uptake is characterized by saturation kinetics (S)0.5 = 20 microM, Vmax = 117 nmoles/min/g liver, cooperatively of mersalyl binding sites, stimulation by extracellular sodium and temperature dependence. Uptake of mersalyl into basolateral membrane vesicles also demonstrates characteristics of a carrier-mediated transport, dependence on extravesicular sodium, cooperativity of mersalyl binding sites, temperature dependence and trans-stimulation by intravesicular non-radioactive mersalyl. Uptake was found to be inhibited by alpha-naphthylacetic acid and mercapto group reagents, suggesting involvement of mercapto groups on the carrier and a binding site for carboxylic anions. Data from the isolated perfused liver and from isolated basolateral vesicles suggest that mersalyl uptake into the liver is carrier mediated. Uptake mechanism and driving forces appear analogous to those for the uptake of chemically related compounds such as taurocholic acid. It is, therefore, speculated that mersalyl may be transported by carrier molecules which accept various chemically unrelated compounds.
Organic mercury is absorbed primarily in the gastrointestinal tract, followed by distribution throughout the body via the bloodstream. Organic mercury forms a complex with free cysteine and the cysteine and sulfhydryl groups on proteins such as hemoglobin (Hgb). These complexes function to mimic methionine and, as a result, are transported throughout the body. This includes travel across the blood-brain barrier and across the placenta.
Mersalyl is a mercurial diuretic which acts on the renal tubules, increasing the excretion of sodium and chloride, in approximately equal amounts, and of water. As a result, blood pressure and edema is markedly decreased. High-affinity binding of the divalent mercuric ion to thiol or sulfhydryl groups of proteins is believed to be the major mechanism for the activity of mercury. Through alterations in intracellular thiol status, mercury can promote oxidative stress, lipid peroxidation, mitochondrial dysfunction, and changes in heme metabolism. Mercury is known to bind to microsomal and mitochondrial enzymes, resulting in cell injury and death. For example, mercury is known to inhibit aquaporins, halting water flow across the cell membrane. It also inhibits the protein LCK, which causes decreased T-cell signaling and immune system depression. Mercury is also believed to inhibit neuronal excitability by acting on the postsynaptic neuronal membrane. It also affects the nervous system by inhibiting protein kinase C and alkaline phosphatase, which impairs brain microvascular formation and function, as well as alters the blood-brain barrier. Organic mercury exhibits developmental effects by binding to tubulin, which prevents microtubule assembly and causes mitotic inhibition. In addition, mercury produces an autoimmune response, likely by modification of major histocompatibility complex (MHC) class II molecules, self-peptides, T-cell receptors, or cell-surface adhesion molecules.