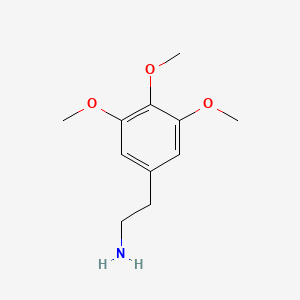

1. 3,4,5-trimethoxyphenethylamine
2. Mezcalin
3. Peyote
4. Trimethoxyphenethylamine
1. Mescalin
2. Tmpea
3. 3,4,5-trimethoxyphenethylamine
4. 54-04-6
5. Mezcaline
6. 2-(3,4,5-trimethoxyphenyl)ethanamine
7. 3,4,5-trimethoxybenzeneethanamine
8. 3,4,5-trimethoxyphenylethylamine
9. Mezcalin
10. Benzeneethanamine, 3,4,5-trimethoxy-
11. Mescalina
12. Mezcalina
13. Meskalin
14. Mescline
15. Mezcline
16. Phenethylamine, 3,4,5-trimethoxy-
17. Nsc 30419
18. Ethane, 1-amino-2-(3,4,5-trimethoxyphenyl)-
19. 2-(3,4,5-trimethoxy-phenyl)-ethylamine
20. Nsc-30419
21. Chembl26687
22. Rho99102vc
23. 1-amino-2-(3,4,5-trimethoxyphenyl)ethane
24. Chebi:28346
25. 2-(3,4,5-trimethoxyphenyl)ethylamine
26. Mescalin [german]
27. Ncgc00247674-01
28. Einecs 200-190-7
29. Brn 1374088
30. Unii-rho99102vc
31. Dea No. 7381
32. 3,4,5-trimethoxyphenethyl-amine
33. Hsdb 7503
34. Mescaline [mi]
35. Mescaline [hsdb]
36. Mescaline [mart.]
37. Constituent Of Peyote Cacti
38. Mescaline [who-dd]
39. 3,5-trimethoxyphenethylamine
40. Oprea1_166025
41. Schembl34190
42. Divk1c_000984
43. 3,5-trimethoxyphenylethylamine
44. Ethane,4,5-trimethoxyphenyl)-
45. Zinc1689
46. 3,4,5-trimethoxy-phenethylamine
47. Kbio1_000984
48. Dtxsid80202303
49. Ninds_000984
50. Benzeneethanamine,4,5-trimethoxy-
51. Wln: Z2r Co1 Do1 Eo1
52. 3,4,5-trimethoxy-b-phenethylamine
53. Nsc30419
54. Bdbm50059891
55. Mfcd00128240
56. (3,4,5-trimethoxy)-benzylmethylamine
57. Akos000277426
58. Sb37575
59. 2-(3,4,5-trimethoxyphenyl)-ethylamine
60. Idi1_000984
61. 2-(3,4,5-trimethoxyphenyl)-ethyl-amine
62. 2-(3,4,5-trimethoxyphenyl)ethanamine #
63. Phenethylamine, 3,4,5-trimethoxy- (8ci)
64. Benzeneethanamine, 3,4,5-trimethoxy- (9ci)
65. C06546
66. Q193140
67. J-505719
| Molecular Weight | 211.26 g/mol |
|---|---|
| Molecular Formula | C11H17NO3 |
| XLogP3 | 0.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 5 |
| Exact Mass | 211.12084340 g/mol |
| Monoisotopic Mass | 211.12084340 g/mol |
| Topological Polar Surface Area | 53.7 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 163 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Hallucinogens
Drugs capable of inducing illusions, hallucinations, delusions, paranoid ideations, and other alterations of mood and thinking. Despite the name, the feature that distinguishes these agents from other classes of drugs is their capacity to induce states of altered perception, thought, and feeling that are not experienced otherwise. (See all compounds classified as Hallucinogens.)
Serotonin Receptor Agonists
Endogenous compounds and drugs that bind to and activate SEROTONIN RECEPTORS. Many serotonin receptor agonists are used as ANTIDEPRESSANTS; ANXIOLYTICS; and in the treatment of MIGRAINE DISORDERS. (See all compounds classified as Serotonin Receptor Agonists.)
Mescaline binds to liver proteins but not to plasma proteins. It is rapidly and widely distributed into the peripheral tissues. The volume of distribution is not specifically known but is believed to be on the order of several L/kg.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 1706
Sixty percent of mescaline is excreted unchanged in a 24 hour urine, and the rest is excreted as metabolites.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 1706
Peyote is rapidly absorbed after ingestion. The onset of action is between 30 minutes and 2 hours, and peak blood levels occur 2 hours after ingestion. The duration of the effect usually ranges from 6 to 12 hours but may last up to 14 hours. /Peyote/
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 1706
Rats of 1, 4, 8, 12, 20, and 60 days postnatal age were injected ip with (14)C-mescaline (50 nCi/g). The levels of mescaline and its deaminated metabolite, 3,4,5-trimethoxyphenylacetic acid, were examined in the brain, liver, heart, spleen, lung, and kidney at 30, 60, 90, and 120 min. Mescaline was rapidly taken up by all the organs examined. In general, the organs of younger rats accumulated much larger amounts than those of adult animals. Brain concentrated the lowest amounts in comparison with other tissues. In the brain, the uptake was the highest in 1-day-old rats and decreased with age. The disappearance of mescaline in various organs was comparatively slower in younger animals than in 20-day or older rats. Rats immediately after birth and uptake was the highest in 1-day-old rats and decreased with age. The disappearance of mescaline in various organs was comparatively slower in younger animals than in 20-day or older rats. Rats immediately after birth and up to 20 days of age metabolized mescaline less efficiently than adults. From the data, it appears that the blood-brain barrier for mescaline develops gradually with age but is not completely impermeable in adults.
PMID:236161 Shah NS et al; Drug Metab Dispos 3 (2): 74-9 (1975)
It is metabolized in the liver by mescaline oxidase into numerous inactive metabolites. These include 3,4,5-trimethoxyphenylacetic acid; 3,4,5-trimethoxybenzoic acid; 3,4-dihydroxy-5-methoxy-phenyl-lacetyl glutamine; 3-hydroxy-4,5-dimethoxyphenylethylamine; N-acetylmescaline; and N-acetyl-3,4-dimethoxy-5-hydroxyphenylethylamine.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 1706
Metabolism of mescaline by several rabbit tissues was examined in vitro. Mescaline-oxidizing activity (micromoles/ per milligram of protein/15 min) of lung homogenates was 4 times greater than that of either liver or kidney. Brain and plasma each had comparatively little capacity to metabolize mescaline. Mescaline metabolism in vitro was sensitive to inhibition by semicarbazide. Removal of mescaline from the medium perfusing the isolated rabbit lung was explained by intrapulmonary metabolism. Semicarbazide (10(-3) M pargyline. Semicarbazide-treated lungs accumulated more mescaline than did untreated lungs. Mescaline efflux from lung was slower than that of its metabolite. These results indicate that the intact lung removes perfused mescaline and may be important in the disposition of circulating mescaline in vivo.
PMID:839444 Roth RA Jr et al; J Pharmacol Exp Ther 200 (2): 394-401(1977)
Hallucinogens, including mescaline, psilocybin, and lysergic acid diethylamide (LSD), profoundly affect perception, cognition, and mood. All known drugs of this class are 5-HT(2A) receptor (2AR) agonists, yet closely related 2AR agonists such as lisuride lack comparable psychoactive properties. Why only certain 2AR agonists are hallucinogens and which neural circuits mediate their effects are poorly understood. By genetically expressing 2AR only in cortex, we show that 2AR-regulated pathways on cortical neurons are sufficient to mediate the signaling pattern and behavioral response to hallucinogens. Hallucinogenic and nonhallucinogenic 2AR agonists both regulate signaling in the same 2AR-expressing cortical neurons. However, the signaling and behavioral responses to the hallucinogens are distinct. While lisuride and LSD both act at 2AR expressed by cortex neurons to regulate phospholipase C, LSD responses also involve pertussis toxin-sensitive heterotrimeric G(i/o) proteins and Src. These studies identify the long-elusive neural and signaling mechanisms responsible for the unique effects of hallucinogens.
PMID:17270739 Gonzalez-Maeso J et al; Neuron 53 (3): 439-52 (2007)
The mechanism of action of mescaline is unknown. Hallucinogenic effects are believed to be due to stimulation of serotonin and dopamine receptors in the CNS.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 1706
Mescaline (3,4,5-trimethoxyphenylethylamine; MES) and its analogs, anhalinine (ANH) and methylenemescaline trimer (MMT) were investigated, using sciatic-sartorius preparations of the frog and cortical tissue from the rat. The effects of MES and its analogs were examined with respect to muscle twitch, resting membrane potential and nicotinic receptor binding. Mescaline and its analogs (10-100 microM) blocked both directly and neurally evoked twitches but their effects on neurally evoked twitches were greater than those on directly evoked twitches. Mescaline, ANH and MMT decreased amplitude of the miniature endplate and endplate potentials, decreased acetylcholine (ACh) quantal content, hyperpolarized the resting membrane potential and prolonged duration of the action potential. They did not significantly displace the binding of [125I]-alpha-bungarotoxin (alpha-BTX) to nicotinic receptors, at concentrations which blocked neuromuscular transmission. These Mescaline (3,4,5-trimethoxyphenylethylamine; MES) and its analogs, anhalinine (ANH) and methylenemescaline trimer (MMT) were investigated, using sciatic-sartorius preparations of the frog and cortical tissue from the rat. The effects of MES and its analogs were examined with respect to muscle twitch, resting membrane potential and nicotinic receptor binding. Mescaline and its analogs (10-100 microM) blocked both directly and neurally evoked twitches but their effects on neurally evoked twitches were greater than those on directly evoked twitches. Mescaline, ANH and MMT decreased amplitude of the miniature endplate and endplate potentials, decreased acetylcholine (ACh) quantal content, hyperpolarized the resting membrane potential and prolonged duration of the action potential. They did not significantly displace the binding of [125I]-alpha-bungarotoxin (alpha-BTX) to nicotinic receptors, at concentrations which blocked neuromuscular transmission. These results suggest that MES and its analogs inhibit cholinergic neuromuscular transmission by blocking release of ACh; they also affect K+ conductance.
PMID:8383816 Ghansah E et al; Neuropharmacology 32 (2): 169-74 (1993)