


1. Aramine
2. Araminol
3. Bitartrate, Metaraminol
4. Hydroxyphenylpropanolamine
5. Isophenylephrine
6. M Hydroxynorephedrine
7. M Hydroxyphenylpropanolamine
8. M-hydroxynorephedrine
9. M-hydroxyphenylpropanolamine
10. Meta Hydroxynorephedrine
11. Meta-hydroxynorephedrine
12. Metaradrin
13. Metaraminol
14. Metaraminol Bitartrate (1:1)
15. Metaraminol Tartrate
16. Tartrate, Metaraminol
1. 33402-03-8
2. Metaraminol Tartrate
3. Metaraminol (+)-bitartrate Salt
4. Metaraminol (tartrate)
5. (-)-metaraminol Bitartrate
6. Metaradrine Tartrate
7. Zc4202m9p3
8. Nsc-758425
9. Araminum
10. Benzenemethanol, A-[(1s)-1-aminoethyl]-3-hydroxy-, (ar)-,(2r,3r)-2,3-dihydroxybutanedioate (1:1) (salt)
11. Levicor
12. Metaramin
13. Levicor Ampullen
14. Araminum Ampullen
15. 3-[(1r,2s)-2-amino-1-hydroxypropyl]phenol;(2r,3r)-2,3-dihydroxybutanedioic Acid
16. L-metraminol Bitartrate
17. Metaradrine Bitartrate
18. Aramine (sympathominetic)
19. Metaraminol Tartrate (1:1)
20. Unii-zc4202m9p3
21. Ims Metaraminol Bitartrat Ampullen
22. Metaraminol (r,r)-hydrogentartrat
23. Metaraminol Bitartrate [usp:jan]
24. Aramine (tn)
25. Einecs 251-502-3
26. Metaraminol Bitatrate
27. Prestwick3_000197
28. Metaraminol Bitartrate Salt
29. (-)-m-hydroxyphenylpropanolamine Bitartrate Salt
30. Schembl40982
31. Bspbio_000153
32. Bspbio_003506
33. Bpbio1_000169
34. 1-alpha-(1-aminoethyl)-m-hydroxybenzyl Alcohol Bitartrate
35. (-)-alpha-(1-aminoethyl)-m-hydroxybenzyl Alcohol Bitartrate
36. Hms2093a03
37. Hms3885j21
38. L-1-(m-hydroxyphenyl)-2-amino-1-propanol-d-hydrogen Tartrate
39. Metaraminol Bitartrate (jan/usp)
40. Pharmakon1600-01503251
41. Metaraminol Bitartrate [mi]
42. Ex-a1348
43. Hy-b1116
44. Metaraminol Bitartrate [jan]
45. 1-alpha-(1-aminoethyl)-m-hydroxybenzyl Alcohol Hydrogen D-tartrate
46. Metaraminol Tartrate [mart.]
47. Nsc758425
48. Metaraminol Bitartrate [vandf]
49. Metaraminol Tartrate [who-dd]
50. (-)-alpha-(1-aminoethyl)-m-hydroxybenzyl Alcohol Tartrate (1:1) (salt)
51. Akos037515726
52. Ccg-213141
53. Cs-4715
54. Metaraminol Bitartrate [usp-rs]
55. Nsc 758425
56. Benzyl Alcohol, Alpha-(1-aminoethyl)-m-hydroxy-, (-)-, Tartrate (1:1) (salt)
57. Ac-33541
58. Benzenemethanol, Alpha-(1-aminoethyl)-3-hydroxy-, (r-(r*,s*))-, (r-(r*,r*))-2,3-dihydroxybutanedioate (1:1) (salt)
59. Bm166414
60. Metaraminol Bitartrate [orange Book]
61. Metaraminol Bitartrate (hydrogen L-tartrate)
62. Metaraminol Bitartrate [usp Impurity]
63. Metaraminol Bitartrate [usp Monograph]
64. D01019
65. F18205
66. Metaraminol Bitartrate, (r-(r*,s*))-
67. 402m038
68. Sr-05000001611
69. J-019180
70. Sr-05000001611-1
71. Q27107333
72. Metaraminol Bitartrate, United States Pharmacopeia (usp) Reference Standard
73. (-)-.alpha.-(1-aminoethyl)-m-hydroxybenzyl Alcohol Tartrate (1:1) (salt)
74. 3-((1r,2s)-2-amino-1-hydroxypropyl)phenol (2r,3r)-2,3-dihydroxysuccinate
75. Benzyl Alcohol, Alpha-(1-aminoethyl)-m-hydroxy-, Tartrate (1:1), D-(-)-
76. Benzenemethanol, Alpha-((1s)-1-aminoethyl)-3-hydroxy-, (alphar)-, (2r,3r)-2,3-dihydroxybutanedioate (1:1)
1. (-)-metaraminol
2. Hydroxynorephedrine
3. L-metaraminol
4. Metaradrine
5. M-hydroxypropadrine
6. Pressonex
7. Metaraminol
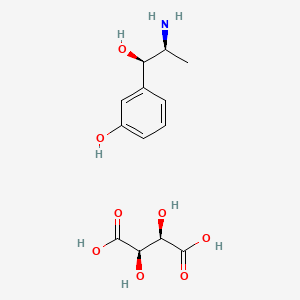
| Molecular Weight | 317.29 g/mol |
|---|---|
| Molecular Formula | C13H19NO8 |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 5 |
| Exact Mass | 317.11106656 g/mol |
| Monoisotopic Mass | 317.11106656 g/mol |
| Topological Polar Surface Area | 182 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 274 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Metaraminol bitartrate |
| Active Ingredient | Metaraminol bitartrate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 10mg base/ml |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
| 2 of 2 | |
|---|---|
| Drug Name | Metaraminol bitartrate |
| Active Ingredient | Metaraminol bitartrate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 10mg base/ml |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
Sympathomimetics
Drugs that mimic the effects of stimulating postganglionic adrenergic sympathetic nerves. Included here are drugs that directly stimulate adrenergic receptors and drugs that act indirectly by provoking the release of adrenergic transmitters. (See all compounds classified as Sympathomimetics.)
Vasoconstrictor Agents
Drugs used to cause constriction of the blood vessels. (See all compounds classified as Vasoconstrictor Agents.)
Adrenergic alpha-1 Receptor Agonists
Compounds that bind to and activate ADRENERGIC ALPHA-1 RECEPTORS. (See all compounds classified as Adrenergic alpha-1 Receptor Agonists.)