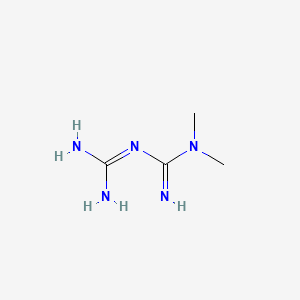

1. Dimethylbiguanidine
2. Dimethylguanylguanidine
3. Glucophage
4. Hcl, Metformin
5. Hydrochloride, Metformin
6. Metformin Hcl
7. Metformin Hydrochloride
1. 657-24-9
2. 1,1-dimethylbiguanide
3. N,n-dimethylimidodicarbonimidic Diamide
4. Fluamine
5. Metformine
6. Metiguanide
7. Dimethylbiguanide
8. Haurymelin
9. Flumamine
10. Gliguanid
11. Melbin
12. Imidodicarbonimidic Diamide, N,n-dimethyl-
13. N,n-dimethylbiguanide
14. N1,n1-dimethylbiguanide
15. Dmgg
16. Nndg
17. N,n-dimethyldiguanide
18. 3-(diaminomethylidene)-1,1-dimethylguanidine
19. 1,1-dimethyl Biguanide
20. Biguanide, 1,1-dimethyl-
21. La-6023
22. Metformin Extended Release
23. Chembl1431
24. Chebi:6801
25. Glifage
26. Islotin
27. Siofor
28. Dimethyldiguanide
29. Diaformin
30. 9100l32l2n
31. Metformina [dcit]
32. Metforminum
33. Metformina
34. Metformina [spanish]
35. Metformine [inn-french]
36. Metforminum [inn-latin]
37. Metformin [usan:inn:ban]
38. Mls000028493
39. [14c]metformin
40. [14c]-metformin
41. 657m249
42. Metformin (usan/inn)
43. Ncgc00016564-01
44. Smr000058277
45. Einecs 211-517-8
46. Cas-1115-70-4
47. Dianben
48. Obimet
49. Dimethylbiguanid
50. Ccris 9321
51. Unii-9100l32l2n
52. N-dimethylbiguanide
53. Dmbg
54. Glucophage (salt/mix)
55. Metformin [inn]
56. N',n'-dimethylbiguanide
57. Metformin [mi]
58. Metformin [usan]
59. Imidodicarbonimidic Diamide-, N,n-dimethyl-
60. Prestwick0_000004
61. Prestwick1_000004
62. Prestwick2_000004
63. Prestwick3_000004
64. Metformin [vandf]
65. Dsstox_cid_3270
66. N,n-dimethylguanylguanidin
67. Metformin [who-dd]
68. Schembl8944
69. Dsstox_rid_76950
70. Dsstox_gsid_23270
71. Bspbio_000007
72. Bspbio_002314
73. Kbiogr_002310
74. Kbioss_002312
75. Cid_14219
76. La 6023 (salt/mix)
77. Bidd:gt0697
78. Spbio_001928
79. Bpbio1_000009
80. Gtpl4503
81. Gtpl4779
82. Schembl9913821
83. Dtxsid2023270
84. Schembl10276396
85. Bdbm57047
86. Kbio2_002310
87. Kbio2_004878
88. Kbio2_007446
89. Kbio3_002790
90. Cmap_000016
91. Hms2089d19
92. Hy-b0627
93. Tox21_302370
94. Bbl012337
95. Bdbm50229665
96. Hsci1_000295
97. Mfcd00242652
98. Nsc813213
99. S5958
100. Stk011633
101. Stl483693
102. Stl484070
103. Zinc12859773
104. Akos000121065
105. Akos005206848
106. Akos015966566
107. Ccg-102605
108. Db00331
109. Nsc-813213
110. 3-carbamimidoyl-1,1-dimethyl-guanidine
111. Ncgc00016564-02
112. Ncgc00016564-03
113. Ncgc00016564-05
114. Ncgc00016564-07
115. Ncgc00188959-01
116. Ncgc00255255-01
117. Ac-32484
118. As-65365
119. Cas-657-24-9
120. Sbi-0206876.p001
121. 1-carbamimidamido-n,n-dimethylmethanimidamide
122. Cs-0009563
123. Ft-0628266
124. A19551
125. C07151
126. D04966
127. D72476
128. Q19484
129. (e)-3-[amino(dimethylamino)methylidene]guanidine
130. 1-[(e)-amino(dimethylamino)methylidene]guanidine
131. W-109589
132. Brd-k79602928-003-04-1
133. Brd-k79602928-003-08-2
134. 3-(diaminomethylene)-1,1-dimethyl-guanidine;hydrochloride
135. 3-[bis(azanyl)methylidene]-1,1-dimethyl-guanidine;hydrochloride
136. Mf8
| Molecular Weight | 129.16 g/mol |
|---|---|
| Molecular Formula | C4H11N5 |
| XLogP3 | -1.3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 2 |
| Exact Mass | 129.10144537 g/mol |
| Monoisotopic Mass | 129.10144537 g/mol |
| Topological Polar Surface Area | 91.5 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 132 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 10 | |
|---|---|
| Drug Name | Fortamet |
| PubMed Health | Metformin (By mouth) |
| Drug Classes | Hypoglycemic |
| Active Ingredient | Metformin hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 500mg; 1gm |
| Market Status | Prescription |
| Company | Andrx Labs |
| 2 of 10 | |
|---|---|
| Drug Name | Glucophage |
| PubMed Health | Metformin (By mouth) |
| Drug Classes | Hypoglycemic |
| Drug Label | GLUCOPHAGE (metformin hydrochloride) Tablets and GLUCOPHAGE XR (metformin hydrochloride) Extended-Release Tablets are oral antihyperglycemic drugs used in the management of type 2 diabetes. Metformin hydrochloride (N,N-dimethylimidodicarbonimidic... |
| Active Ingredient | Metformin hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 850mg; 500mg; 1gm |
| Market Status | Prescription |
| Company | Bristol Myers Squibb |
| 3 of 10 | |
|---|---|
| Drug Name | Glucophage xr |
| PubMed Health | Metformin (By mouth) |
| Drug Classes | Hypoglycemic |
| Drug Label | GLUCOPHAGE (metformin hydrochloride) Tablets and GLUCOPHAGE XR (metformin hydrochloride) Extended-Release Tablets are oral antihyperglycemic drugs used in the management of type 2 diabetes. Metformin hydrochloride (N,N-dimethylimidodicarbonimidic... |
| Active Ingredient | Metformin hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 500mg; 750mg |
| Market Status | Prescription |
| Company | Bristol Myers Squibb |
| 4 of 10 | |
|---|---|
| Drug Name | Glumetza |
| PubMed Health | Metformin (By mouth) |
| Drug Classes | Hypoglycemic |
| Active Ingredient | Metformin hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 500mg; 1gm |
| Market Status | Prescription |
| Company | Santarus |
| 5 of 10 | |
|---|---|
| Drug Name | Riomet |
| Active Ingredient | Metformin hydrochloride |
| Dosage Form | Solution |
| Route | Oral |
| Strength | 500mg/5ml |
| Market Status | Prescription |
| Company | Ranbaxy |
| 6 of 10 | |
|---|---|
| Drug Name | Fortamet |
| PubMed Health | Metformin (By mouth) |
| Drug Classes | Hypoglycemic |
| Active Ingredient | Metformin hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 500mg; 1gm |
| Market Status | Prescription |
| Company | Andrx Labs |
| 7 of 10 | |
|---|---|
| Drug Name | Glucophage |
| PubMed Health | Metformin (By mouth) |
| Drug Classes | Hypoglycemic |
| Drug Label | GLUCOPHAGE (metformin hydrochloride) Tablets and GLUCOPHAGE XR (metformin hydrochloride) Extended-Release Tablets are oral antihyperglycemic drugs used in the management of type 2 diabetes. Metformin hydrochloride (N,N-dimethylimidodicarbonimidic... |
| Active Ingredient | Metformin hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 850mg; 500mg; 1gm |
| Market Status | Prescription |
| Company | Bristol Myers Squibb |
| 8 of 10 | |
|---|---|
| Drug Name | Glucophage xr |
| PubMed Health | Metformin (By mouth) |
| Drug Classes | Hypoglycemic |
| Drug Label | GLUCOPHAGE (metformin hydrochloride) Tablets and GLUCOPHAGE XR (metformin hydrochloride) Extended-Release Tablets are oral antihyperglycemic drugs used in the management of type 2 diabetes. Metformin hydrochloride (N,N-dimethylimidodicarbonimidic... |
| Active Ingredient | Metformin hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 500mg; 750mg |
| Market Status | Prescription |
| Company | Bristol Myers Squibb |
| 9 of 10 | |
|---|---|
| Drug Name | Glumetza |
| PubMed Health | Metformin (By mouth) |
| Drug Classes | Hypoglycemic |
| Active Ingredient | Metformin hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 500mg; 1gm |
| Market Status | Prescription |
| Company | Santarus |
| 10 of 10 | |
|---|---|
| Drug Name | Riomet |
| Active Ingredient | Metformin hydrochloride |
| Dosage Form | Solution |
| Route | Oral |
| Strength | 500mg/5ml |
| Market Status | Prescription |
| Company | Ranbaxy |
Hypoglycemic Agents
National Library of Medicine's Medical Subject Headings. Metformin. Online file (MeSH, 2016). Available from, as of October 28, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
Metformin hydrochloride tablets, USP are indicated as an adjunct to diet and exercise to improve glycemic control in adults and children with type 2 diabetes mellitus. /Included in US product label/
NIH; DailyMed. Current Medication Information for Metformin Hydrochloride Tablet (Updated: December 2015). Available from, as of November 23, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=da99b8c4-f85e-409f-93a8-0e4758d74552
Metformin has been used in the management of metabolic and reproductive abnormalities associated with polycystic ovary syndrome. However, adequate and well-controlled clinical trials evaluating metformin therapy for polycystic ovary syndrome remain limited, particularly regarding long-term efficacy, and available data are conflicting regarding the benefits of the drug in ameliorating various manifestations of the condition. /NOT included in US product label/
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3194
Metformin is commercially available in fixed combination with glyburide or glipizide for use as an adjunct to diet and exercise to improve glycemic control in adults with diabetes mellitus; such fixed-combination preparations may be used as initial therapy in patients whose hyperglycemia cannot be controlled by diet and exercise alone, or as second-line therapy in patients who do not achieve adequate control of hyperglycemia with metformin or sulfonylurea monotherapy. A thiazolidinedione may be added to metformin in fixed combination with glyburide in patients who have inadequate glycemic control with fixed-combination therapy.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3191
For more Therapeutic Uses (Complete) data for METFORMIN (18 total), please visit the HSDB record page.
/BOXED WARNING/ Lactic Acidosis: Lactic acidosis is a rare, but serious, metabolic complication that can occur due to metformin accumulation during treatment with metformin; when it occurs, it is fatal in approximately 50% of cases. Lactic acidosis may also occur in association with a number of pathophysiologic conditions, including diabetes mellitus, and whenever there is significant tissue hypoperfusion and hypoxemia. Lactic acidosis is characterized by elevated blood lactate levels (>5 mmol/L), decreased blood pH, electrolyte disturbances with an increased anion gap, and an increased lactate/pyruvate ratio. When metformin is implicated as the cause of lactic acidosis, metformin plasma levels > 5 ug/mL are generally found. The reported incidence of lactic acidosis in patients receiving metformin hydrochloride tablets, USP is very low (approximately 0.03 cases/1000 patient-years, with approximately 0.015 fatal cases/1000 patient-years). In more than 20,000 patient-years exposure to metformin in clinical trials, there were no reports of lactic acidosis. Reported cases have occurred primarily in diabetic patients with significant renal insufficiency, including both intrinsic renal disease and renal hypoperfusion, often in the setting of multiple concomitant medical/surgical problems and multiple concomitant medications. Patients with congestive heart failure requiring pharmacologic management, in particular those with unstable or acute congestive heart failure who are at risk of hypoperfusion and hypoxemia are at increased risk of lactic acidosis. The risk of lactic acidosis increases with the degree of renal dysfunction and the patient's age. The risk of lactic acidosis may, therefore, be significantly decreased by regular monitoring of renal function in patients taking metformin and by use of the minimum effective dose of metformin. In particular, treatment of the elderly should be accompanied by careful monitoring of renal function. Metformin hydrochloride tablets, USP treatment should not be initiated in patients = 80 years of age unless measurement of creatinine clearance demonstrates that renal function is not reduced, as these patients are more susceptible to developing lactic acidosis. In addition, metformin should be promptly withheld in the presence of any condition associated with hypoxemia, dehydration or sepsis. Because impaired hepatic function may significantly limit the ability to clear lactate, metformin should generally be avoided in patients with clinical or laboratory evidence of hepatic disease. Patients should be cautioned against excessive alcohol intake, either acute or chronic, when taking metformin hydrochloride tablets, USP since alcohol potentiates the effects of metformin hydrochloride tablets, USP on lactate metabolism. In addition, metformin should be temporarily discontinued prior to any intravascular radiocontrast study and for any surgical procedure. The onset of lactic acidosis often is subtle, and accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, increasing somnolence and nonspecific abdominal distress. There may be associated hypothermia, hypotension and resistant bradyarrhythmias with more marked acidosis. The patient and the patient's physician must be aware of the possible importance of such symptoms and the patient should be instructed to notify the physician immediately if they occur. Metformin hydrochloride tablets, USP should be withdrawn until the situation is clarified. Serum electrolytes, ketones, blood glucose and, if indicated, blood pH, lactate levels and even blood metformin levels may be useful. Once a patient is stabilized on any dose level of metformin, gastrointestinal symptoms, which are common during initiation of therapy, are unlikely to be drug related. Later occurrence of gastrointestinal symptoms, could be due to lactic acidosis or other serious disease. Levels of fasting venous plasma lactate above the upper limit of normal but less than 5 mmol/L in patients taking metformin do not necessarily indicate impending lactic acidosis and may be explainable by other mechanisms, such as poorly controlled diabetes or obesity, vigorous physical activity or technical problems in sample handling. Lactic acidosis should be suspected in any diabetic patient with metabolic acidosis lacking evidence of ketoacidosis (ketonuria and ketonemia). Lactic acidosis is a medical emergency that must be treated in a hospital setting. In a patient with lactic acidosis who is taking metformin, the drug should be discontinued immediately and general supportive measures promptly instituted. Because metformin hydrochloride tablets, USP are dialyzable (with a clearance of up to 170 mL/min under good hemodynamic conditions), prompt hemodialysis is recommended to correct the acidosis and remove the accumulated metformin. Such management often results in prompt reversal of symptoms and recovery.
NIH; DailyMed. Current Medication Information for Metformin Hydrochloride Tablet (Updated: December 2015). Available from, as of November 23, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=da99b8c4-f85e-409f-93a8-0e4758d74552
Accumulation of metformin may occur in patients with renal impairment, and such accumulation rarely can result in lactic acidosis, a serious, potentially fatal metabolic disease. The risk of developing lactic acidosis is much lower (e.g., 10-fold lower) with metformin than with phenformin (no longer commercially available in the US). However, lactic acidosis constitutes a medical emergency requiring immediate hospitalization and treatment; in such cases, metformin should be discontinued and general supportive therapy (e.g., volume expansion, diuresis) should be initiated immediately. Prompt hemodialysis also is recommended. Lactic acidosis is characterized by elevated blood lactate concentrations (exceeding 45 mg/dL), decreased blood pH (less than 7.35), electrolyte disturbances with an increased anion gap, and an increased lactate/pyruvate ratio. Lactic acidosis also may occur in association with a variety of pathophysiologic conditions, including diabetes mellitus, and whenever substantial tissue hypoperfusion and hypoxemia exist. Approximately 50% of cases of metformin-associated lactic acidosis have been reported to be fatal. However, it has been suggested that in such cases of lactic acidosis not accompanied by conditions predisposing to tissue anoxia (e.g., heart failure, renal or pulmonary disease), techniques for the elimination of metformin from the body may allow recovery rates exceeding 80%.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3197-8
Urinary tract infection has been reported in 8 or 1.1% of patients receiving metformin alone or in fixed combination with glipizide, respectively. Hypertension has been reported in 5.6 or 2.9-3.5% of patients receiving metformin alone or in fixed combination with glipizide, respectively. Musculoskeletal pain has been reported in 6.7 or 8% of patients receiving metformin alone or in fixed combination with glipizide, respectively. Severe acute hepatitis associated with marked elevations in serum hepatic aminotransferase values and cholestasis has been reported following initiation of metformin therapy in a patient receiving glipizide and enalapril. Accidental injury was reported in 7.3 or 5.6% of patients receiving metformin as an extended-release tablet preparation (Fortamet) or as conventional tablets, respectively.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3199
Pneumonitis with vasculitis has been reported rarely with concomitant metformin and oral sulfonylurea (e.g., glyburide) therapy. Upper respiratory tract infection was reported in 16.3 or 17.3% of patients receiving metformin or metformin in fixed combination with glyburide, respectively. Upper respiratory tract infection was reported in 8.5 or 8.1-9.9% of patients receiving metformin or metformin in fixed combination with glipizide, respectively, as initial therapy for type 2 diabetes mellitus. Upper respiratory tract infection was reported in 10.7 or 10.3% of patients receiving metformin or metformin in fixed combination with glipizide, respectively, as second-line therapy for type 2 diabetes mellitus. Upper respiratory tract infection was reported in 5.2 or 6.2% of patients receiving metformin or metformin combined with sitagliptin, respectively, in clinical trials. Rhinitis was reported in 4.2 or 5.6% of patients receiving metformin as an extended-release tablet preparation (Fortamet) or as conventional tablets, respectively. Infection was reported in 20.5 or 20.9% of patients receiving an extended-release tablet preparation (Fortamet) or conventional tablets, respectively.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3199
For more Drug Warnings (Complete) data for METFORMIN (23 total), please visit the HSDB record page.
**Metformin tablet** Metformin is indicated as an adjunct to diet and exercise to increase glycemic control in _adults and pediatric patients_ 10 years of age and older diagnosed with type 2 diabetes mellitus. **Metformin extended-release tablet (XR)** The extended-release form is indicated as an adjunct to diet and exercise to improve glycemic control in only _adults_ with type 2 diabetes mellitus. Safety in children has not been determined to this date. An extended-release combination product containing empagliflozin, linagliptin, and metformin was approved by the FDA in January 2020 for the improvement of glycemic control in adults with type 2 diabetes mellitus when used adjunctively with diet and exercise.
FDA Label
**General effects** Insulin is an important hormone that regulates blood glucose levels. Type II diabetes is characterized by a decrease in sensitivity to insulin, resulting in eventual elevations in blood glucose when the pancreas can no longer compensate. In patients diagnosed with type 2 diabetes, insulin no longer exerts adequate effects on tissues and cells (called insulin resistance) and insulin deficiency may also be present. Metformin reduces liver (hepatic) production of glucose, decreases the intestinal absorption of glucose, and enhances insulin sensitivity by increasing both peripheral glucose uptake and utilization. In contrast with drugs of the _sulfonylurea_ class, which lead to hyperinsulinemia, the secretion of insulin is unchanged with metformin use. **Effect on fasting plasma glucose (FPG) and Glycosylated hemoglobin (HbA1c)** HbA1c is an important periodic measure of glycemic control that is used to monitor diabetic patients. Fasting plasma glucose is also a useful and important measure of glycemic control. In a 29-week clinical trial of subjects diagnosed with type II diabetes, metformin decreased the fasting plasma glucose levels by an average of 59 mg/dL from baseline, compared to an average increase of 6.3 mg/dL from baseline in subjects taking a placebo. Glycosylated hemoglobin (HbA1c) was decreased by about 1.4% in subjects receiving metformin, and increased by 0.4% in subjects receiving placebo only.
Hypoglycemic Agents
Substances which lower blood glucose levels. (See all compounds classified as Hypoglycemic Agents.)
A10BA02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A10BA02
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
A - Alimentary tract and metabolism
A10 - Drugs used in diabetes
A10B - Blood glucose lowering drugs, excl. insulins
A10BA - Biguanides
A10BA02 - Metformin
Absorption
**Regular tablet absorption** The absolute bioavailability of a metformin 500 mg tablet administered in the fasting state is about 50%-60%. Single-dose clinical studies using oral doses of metformin 500 to 1500 mg and 850 to 2550 mg show that there is a lack of dose proportionality with an increase in metformin dose, attributed to decreased absorption rather than changes in elimination. At usual clinical doses and dosing schedules of metformin, steady-state plasma concentrations of metformin are achieved within 24-48 hours and are normally measured at <1 g/mL. **Extended-release tablet absorption** After a single oral dose of metformin extended-release, Cmax is reached with a median value of 7 hours and a range of between 4 and 8 hours. Peak plasma levels are measured to be about 20% lower compared to the same dose of regular metformin, however, the extent of absorption of both forms (as measured by area under the curve - AUC), are similar. **Effect of food** Food reduces the absorption of metformin, as demonstrated by about a 40% lower mean peak plasma concentration (Cmax), a 25% lower area under the plasma concentration versus time curve (AUC), and a 35-minute increase in time to peak plasma concentration (Tmax) after ingestion of an 850 mg tablet of metformin taken with food, compared to the same dose administered during fasting. Though the extent of metformin absorption (measured by the area under the curve - AUC) from the metformin extended-release tablet is increased by about 50% when given with food, no effect of food on Cmax and Tmax of metformin is observed. High and low-fat meals exert similar effects on the pharmacokinetics of extended-release metformin.
Route of Elimination
This drug is substantially excreted by the kidney. Renal clearance of metformin is about 3.5 times higher than creatinine clearance, which shows that renal tubular secretion is the major route of metformin elimination. After oral administration, about 90% of absorbed metformin is eliminated by the kidneys within the first 24 hours post-ingestion.
Volume of Distribution
The apparent volume of distribution (V/F) of metformin after one oral dose of metformin 850 mg averaged at 654 358 L.
Clearance
Renal clearance is about 3.5 times greater than creatinine clearance, which indicates that tubular secretion is the major route of metformin elimination. Following oral administration, approximately 90% of the absorbed drug is eliminated via the renal route within the first 24 hours.
Metformin is slowly and incompletely absorbed from the GI tract, mainly from the small intestine; absorption is complete within 6 hours. The absolute oral bioavailability of the drug under fasting conditions is reported to be approximately 50-60% with metformin hydrochloride doses of 0.5-1.5 g; binding of the drug to the intestinal wall may explain the difference between the amount of drug absorbed (as determined by the urinary and fecal excretion of unchanged drug) and the amount bioavailable in some studies. In single-dose studies with metformin hydrochloride conventional tablets doses of 0.5-1.5 g or 0.85-2.55 g, plasma metformin concentrations did not increase in proportion to increasing doses, suggesting an active saturable absorption process. Similarly, in single-dose studies with an extended-release tablet preparation (Glumetza) at doses of 0.5-2.5 g, plasma metformin concentrations did not increase in proportion to increasing doses. At steady state after administration of a metformin hydrochloride extended-release tablet preparation (Glucophage XR), the AUC and peak plasma concentrations were not dose proportional within the range of 0.5-2 g. However, limited data from studies in animals and in human intestinal cell cultures suggest that transepithelial transfer of metformin in the intestine may occur through a passive, nonsaturable mechanism, possibly involving a paracellular route. In several studies with another metformin hydrochloride extended-release tablet preparation (Fortamet) using doses of 1-2.5 g, metformin exposure was dose-related.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3202-3
Following oral administration of metformin hydrochloride (0.5-1.5 g) as conventional tablets in healthy individuals or in patients with type 2 diabetes mellitus, plasma concentrations decline in a triphasic manner. Following multiple-dose administration of metformin hydrochloride (500 mg twice daily for 7-14 days) as conventional tablets in a limited number of patients with type 2 diabetes mellitus, peak plasma concentrations remained unchanged, but trough drug concentrations were higher than with single-dose administration, suggesting some drug accumulation in a peripheral tissue compartment. No accumulation of metformin appears to occur following repeated oral doses of the drug as extended-release tablets. The principal plasma elimination half-life of metformin averages approximately 6.2 hours; 90% of the drug is cleared within 24 hours in patients with normal renal function. The decline in plasma metformin concentrations is slower after oral than after IV administration of the drug, indicating that elimination is absorption rate-limited. Urinary excretion data and data from whole blood indicate a slower terminal-elimination phase half-life of 8-20 hours (e.g., 17.6 hours)1 suggesting that the erythrocyte mass may be a compartment of distribution.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3203
Metformin is distributed rapidly in animals and humans into peripheral body tissues and fluids, particularly the GI tract; the drug also appears to distribute slowly into erythrocytes and into a deep tissue compartment (probably GI tissues). The highest tissue concentrations of metformin (at least 10 times the plasma concentration) occur in the GI tract (e.g., esophagus, stomach, duodenum, jejunum, ileum), with lower concentrations (twice the plasma concentration) occurring in kidney, liver, and salivary gland tissue. The drug distributes into salivary glands with a half-life of about 9 hours. Metformin concentrations in saliva are tenfold lower than those in plasma and may be responsible for the metallic taste reported in some patients receiving the drug. Any local effect of metformin on glucose absorption in the GI tract may be associated with the relatively high GI concentrations of the drug compared with those in other tissues. It is not known whether metformin crosses the blood-brain barrier or the placenta in humans or if the drug is distributed into human milk; however, in lactating rats, metformin is distributed into breast milk at levels comparable to those in plasma.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3203
Renal clearance is approximately 3.5 times greater than creatinine clearance, indicating that tubular secretion is the principal route of metformin elimination. Following a single 850-mg oral dose of metformin hydrochloride, renal clearance averaged 552, 491, or 412 mL/minute in nondiabetic adults, diabetic adults, or healthy geriatric individuals, respectively. Renal impairment results in increased peak plasma concentrations of metformin, a prolonged time to peak plasma concentration, and a decreased volume of distribution. Renal clearance is decreased in patients with renal impairment (as measured by decreases in creatinine clearance) and, apparently because of reduced renal function with age, in geriatric individuals. In geriatric individuals, decreased renal and plasma clearance of metformin also results in increased plasma concentrations of the drug; volume of distribution remains unaffected.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3203
For more Absorption, Distribution and Excretion (Complete) data for METFORMIN (12 total), please visit the HSDB record page.
Intravenous studies using a single dose of metformin in normal subjects show that metformin is excreted as unchanged drug in the urine and does not undergo hepatic metabolism (no metabolites have been identified in humans) or biliary excretion.
Metformin is not metabolized in the liver or GI tract and is not excreted in bile; no metabolites of the drug have been identified in humans.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3203
Approximately 6.2 hours in the plasma and in the blood, the elimination half-life is approximately 17.6 hours, suggesting that the erythrocyte mass may be a compartment of distribution.
The principal plasma elimination half-life of metformin averages approximately 6.2 hours ... .
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3203
The drug distributes into salivary glands with a half-life of about 9 hours.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3203
Metformin's mechanisms of action are unique from other classes of oral antihyperglycemic drugs. Metformin decreases blood glucose levels by decreasing hepatic glucose production (gluconeogenesis), decreasing the intestinal absorption of glucose, and increasing insulin sensitivity by increasing peripheral glucose uptake and utilization. It is well established that metformin inhibits mitochondrial complex I activity, and it has since been generally postulated that its potent antidiabetic effects occur through this mechanism. The above processes lead to a decrease in blood glucose, managing type II diabetes and exerting positive effects on glycemic control. After ingestion, the organic cation transporter-1 (OCT1) is responsible for the uptake of metformin into hepatocytes (liver cells). As this drug is positively charged, it accumulates in cells and in the mitochondria because of the membrane potentials across the plasma membrane as well as the mitochondrial inner membrane. Metformin inhibits mitochondrial complex I, preventing the production of mitochondrial ATP leading to increased cytoplasmic ADP:ATP and AMP:ATP ratios. These changes activate AMP-activated protein kinase (AMPK), an enzyme that plays an important role in the regulation of glucose metabolism. Aside from this mechanism, AMPK can be activated by a lysosomal mechanism involving other activators. Following this process, increases in AMP:ATP ratio also inhibit _fructose-1,6-bisphosphatase_ enzyme, resulting in the inhibition of gluconeogenesis, while also inhibiting _adenylate cyclase_ and decreasing the production of cyclic adenosine monophosphate (cAMP), a derivative of ATP used for cell signaling. Activated AMPK phosphorylates two isoforms of acetyl-CoA carboxylase enzyme, thereby inhibiting fat synthesis and leading to fat oxidation, reducing hepatic lipid stores and increasing liver sensitivity to insulin. In the intestines, metformin increases anaerobic glucose metabolism in enterocytes (intestinal cells), leading to reduced net glucose uptake and increased delivery of lactate to the liver. Recent studies have also implicated the gut as a primary site of action of metformin and suggest that the liver may not be as important for metformin action in patients with type 2 diabetes. Some of the ways metformin may play a role on the intestines is by promoting the metabolism of glucose by increasing glucagon-like peptide I (GLP-1) as well as increasing gut utilization of glucose. In addition to the above pathway, the mechanism of action of metformin may be explained by other ways, and its exact mechanism of action has been under extensive study in recent years.
Metformin is widely used to treat hyperglycemia. However, metformin treatment may induce intrahepatic cholestasis and liver injury in a few patients with type II diabetes through an unknown mechanism. Here we show that metformin decreases SIRT1 protein levels in primary hepatocytes and liver. Both metformin-treated wild-type C57 mice and hepatic SIRT1-mutant mice had increased hepatic and serum bile acid levels. However, metformin failed to change systemic bile acid levels in hepatic SIRT1-mutant mice. Molecular mechanism study indicates that SIRT1 directly interacts with and deacetylates Foxa2 to inhibit its transcriptional activity on expression of genes involved in bile acids synthesis and transport. Hepatic SIRT1 mutation elevates Foxa2 acetylation levels, which promotes Foxa2 binding to and activating genes involved in bile acids metabolism, impairing hepatic and systemic bile acid homeostasis. Our data clearly suggest that hepatic SIRT1 mediates metformin effects on systemic bile acid metabolism and modulation of SIRT1 activity in liver may be an attractive approach for treatment of bile acid-related diseases such as cholestasis.
PMID:27816442 Chen Q et al; Biochim Biophys Acta 1864 (1):101-112 (2016)
Metformin is antihyperglycemic, not hypoglycemic. It does not cause insulin release from the pancreas and does not cause hypoglycemia, even in large doses. Metformin has no significant effects on the secretion of glucagon, cortisol, growth hormone or somatostatin. Metformin reduces glucose levels primarily by decreasing hepatic glucose production and by increasing insulin action in muscle and fat. ... May decrease plasma glucose by reducing the absorption of glucose from the intestine. /Salt not specified/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1705
Metformin potentiates the effect of insulin by mechanisms not fully understood. Metformin does not stimulate pancreatic beta cells to increase secretion of insulin; insulin secretion must be present for metformin to work properly. It is postulated that metformin decreases hepatic glucose production and improves insulin sensitivity by increasing peripheral glucose uptake and utilization. /Salt not specified/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2004
People with Type 2 diabetes mellitus (T2DM) have reduced bone mineral density and an increased risk of fractures due to altered mesenchymal stem cell (MSC) differentiation in the bone marrow. This leads to a shift in the balance of differentiation away from bone formation (osteogenesis) in favour of fat cell development (adipogenesis). The commonly used anti-diabetic drug, metformin, activates the osteogenic transcription factor Runt-related transcription factor 2 (Runx2), which may suppress adipogenesis, leading to improved bone health. Here we investigate the involvement of the metabolic enzyme, AMP-activated protein kinase (AMPK), in these protective actions of metformin. The anti-adipogenic actions of metformin were observed in multipotent C3H10T1/2 MSCs, in which metformin exerted reciprocal control over the activities of Runx2 and the adipogenic transcription factor, PPARgamma, leading to suppression of adipogenesis. These effects appeared to be independent of AMPK activation but rather through the suppression of the mTOR/p70S6K signalling pathway. Basal AMPK and mTOR/p70S6K activity did appear to be required for adipogenesis, as demonstrated by the use of the AMPK inhibitor, compound C. This observation was further supported by using AMPK knockout mouse embryo fibroblasts (MEFs) where adipogenesis, as assessed by reduced lipid accumulation and expression of the adipogeneic transcription factor, C/EBPbeta, was found to display an absolute requirement for AMPK. Further activation of AMPK in wild type MEFS, with either metformin or the AMPK-specific activator, A769662, was also associated with suppression of adipogenesis. It appears, therefore, that basal AMPK activity is required for adipogenesis and that metformin can inhibit adipogenesis through AMPK-dependent or -independent mechanisms, depending on the cellular context.
PMID:27856330 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5228588 Chen SC et al; Mol Cell Endocrinol. 2016 Nov 14. pii: S0303-7207(16)30470-1. doi: 10.1016/j.mce.2016.11.011. (Epub ahead of print)