

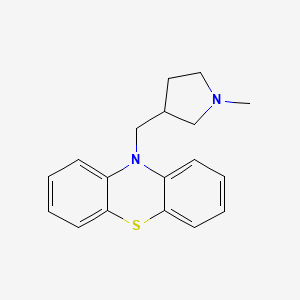

1. Methdilazine Hydrochloride
1. Tacaryl
2. 1982-37-2
3. Methdilazinum
4. Tacazyl
5. Methdilazinum [inn-latin]
6. Metodilazina [inn-spanish]
7. Nci-c60720
8. 10-[(1-methylpyrrolidin-3-yl)methyl]phenothiazine
9. Methdilazine (inn)
10. 10-[(1-methylpyrrolidin-3-yl)methyl]-10h-phenothiazine
11. 10-((1-methyl-3-pyrrolidinyl)methyl)phenothiazine
12. 4q13ly9z8x
13. Chebi:6823
14. Methodilazine
15. Metodilazina
16. 10h-phenothiazine, 10-((1-methyl-3-pyrrolidinyl)methyl)-
17. 10h-phenothiazine, 10-[(1-methyl-3-pyrrolidinyl)methyl]-
18. Tacryl
19. Methdilazine [inn]
20. Nsc169091
21. Mj 5022
22. 10-[(1-methyl-3-pyrrolidinyl)methyl]phenothiazine
23. Phenothiazine, 10-((1-methyl-3-pyrrolidinyl)methyl)-
24. Phenothiazine, 10-[(1-methyl-3-pyrrolidinyl)methyl]-
25. 10-[(1-methyl-3-pyrrolidinyl)methyl]phenothiazine; Mj 5022
26. Tacaryl (tn)
27. Product 5022
28. Einecs 217-841-6
29. Metdilazina
30. Unii-4q13ly9z8x
31. Methdilazine [usp:inn:ban]
32. 10-((1-methyl-3-pyrrolidinyl)methyl)phenothiazine Monohydrochloride
33. Dilosyn (salt/mix)
34. Disyncram (salt/mix)
35. Disyncran (salt/mix)
36. 10h-phenothiazine, 10-((1-methyl-3-pyrrolidinyl)methyl)-, Monohydrochloride
37. Methdilazine [mi]
38. Methdilazine [vandf]
39. Methdilazine [mart.]
40. Methdilazine(200mg)
41. Methdilazine [who-dd]
42. Schembl121507
43. Gtpl7231
44. 10-((1-methyl-3-pyrrolidinyl)methyl)-10h-phenothiazine
45. Chembl1200959
46. Dtxsid6023282
47. Bdbm81470
48. Methdilazine (200 Mg)
49. Methdilazine [orange Book]
50. Hy-b1690
51. 10h-phenothiazine, Tannate (5:1)
52. Cas_14677
53. Nsc_14677
54. Nsc244191
55. Pdsp1_000149
56. Pdsp2_000148
57. Db00902
58. Nsc-244191
59. Nci60_001351
60. Cs-0013673
61. C07175
62. D04979
63. L001130
64. Sr-01000944494
65. Q6665709
66. Sr-01000944494-1
67. 10-[(1-methyl-3-pyrrolidinyl)methyl]-10h-phenothiazine #
| Molecular Weight | 296.4 g/mol |
|---|---|
| Molecular Formula | C18H20N2S |
| XLogP3 | 5.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 296.13471982 g/mol |
| Monoisotopic Mass | 296.13471982 g/mol |
| Topological Polar Surface Area | 31.8 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 339 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used for the symptomatic relief of hypersensitivity reactions and particularly for the control of pruritic skin disorders
In allergic reactions an allergen interacts with and cross-links surface IgE antibodies on mast cells and basophils. Once the mast cell-antibody-antigen complex is formed, a complex series of events occurs that eventually leads to cell-degranulation and the release of histamine (and other chemical mediators) from the mast cell or basophil. Once released, histamine can react with local or widespread tissues through histamine receptors. Histamine, acting on H1-receptors, produces pruritis, vasodilatation, hypotension, flushing, headache, tachycardia, and bronchoconstriction. Histamine also increases vascular permeability and potentiates pain. Methdilazine is a histamine H1 antagonist. It competes with histamine for the normal H1-receptor sites on effector cells of the gastrointestinal tract, blood vessels and respiratory tract. It provides effective, temporary relief of sneezing, watery and itchy eyes, and runny nose due to hay fever and other upper respiratory allergies.
R - Respiratory system
R06 - Antihistamines for systemic use
R06A - Antihistamines for systemic use
R06AD - Phenothiazine derivatives
R06AD04 - Methdilazine
Absorption
Well absorbed in the digestive tract.
Methdilazine binds to the histamine H1 receptor. This blocks the action of endogenous histamine, which subsequently leads to temporary relief of the negative symptoms brought on by histamine.