
1. Carbamate, Guaiphenesin
2. Guaiphenesin Carbamate
3. Lumirelax
4. Ortoton
5. Parabaxin
6. Robaxin
1. 532-03-6
2. Robaxin
3. Lumirelax
4. Metocarbamol
5. Parabaxin
6. Delaxin
7. Perilax
8. Metocarbamolo
9. Etroflex
10. Methocal
11. Metofenia
12. Miolaxene
13. Miorilas
14. Myolaxene
15. Neuraxin
16. Reflexyn
17. Relestrid
18. Romethocarb
19. Surquetil
20. Tresortil
21. Avetil
22. Miowas
23. Robinax
24. Metofenina
25. Forbaxin
26. Guaiacol Glyceryl Ether Carbamate
27. Guaiphenesine Carbamate
28. 2-hydroxy-3-(2-methoxyphenoxy)propyl Carbamate
29. Glycerylguaiacolate Carbamate
30. Glycerylguajacol-carbamat
31. Ahr 85
32. Methocarbamolum
33. Robaxan
34. Robaxine
35. Robaxon
36. Guiacol-gliceriletere Monocarbammato
37. [2-hydroxy-3-(2-methoxyphenoxy)propyl] Carbamate
38. 1,2-propanediol, 3-(2-methoxyphenoxy)-, 1-carbamate
39. 3-(o-methoxyphenoxy)-2-hydroxypropyl Carbamate
40. 3-(o-methoxyphenoxy)-1,2-propanediol 1-carbamate
41. 3-(2-methoxyphenoxy)-1-glyceryl Carbamate
42. Nsc 170960
43. 2-hydroxy-3-(o-methoxyphenoxy)propyl 1-carbamate
44. Methocarbamol (robaxin)
45. Nsc-170960
46. Guaiphenesin Carbamate
47. 1,2-propanediol, 3-(o-methoxyphenoxy)-, 1-carbamate
48. Mls000028610
49. Carbamic Acid, 2-hydroxy-3-(o-methoxyphenoxy)propyl Ester
50. Chebi:77498
51. 125od7737x
52. Robamol
53. Smr000058719
54. Traumacut
55. Dsstox_cid_3286
56. Dsstox_rid_76958
57. Dsstox_gsid_23286
58. Methyocarbamol
59. Metocarbamolo [dcit]
60. Robaxisol
61. Robaxin-750
62. Metocarbamol [inn-spanish]
63. Methocarbamolum [inn-latin]
64. Hsdb 3122
65. Sr-01000000240
66. Einecs 208-524-3
67. 2-hydroxy-3-{[2-(methyloxy)phenyl]oxy}propyl Carbamate
68. Brn 1884446
69. Methacarbamol
70. Unii-125od7737x
71. Ncgc00016492-01
72. Methocarbamol,(s)
73. Cas-532-03-6
74. Methocarbamol [usp:inn:ban:jan]
75. Prestwick_614
76. Delaxin (tn)
77. Robaxin (tn)
78. Mfcd00057662
79. Robaxisol (salt/mix)
80. Spectrum_000997
81. Opera_id_1471
82. Prestwick0_000184
83. Prestwick1_000184
84. Prestwick2_000184
85. Prestwick3_000184
86. Spectrum2_001272
87. Spectrum3_000496
88. Spectrum4_000049
89. Spectrum5_000956
90. Methocarbamol [mi]
91. 2-hydroxy-3-(o-methoxyphenoxy)propyl Carbamate
92. Methocarbamol [inn]
93. Methocarbamol [jan]
94. Methocarbamol [hsdb]
95. Schembl34365
96. Bspbio_000107
97. Bspbio_001991
98. Kbiogr_000517
99. Kbioss_001477
100. Methocarbamol [vandf]
101. 3-(2-methoxyphenoxy)-1,2-propanediol 1-carbamate
102. Mls001148605
103. Divk1c_000434
104. Methocarbamol [mart.]
105. Spectrum1500397
106. Wln: Zvo1yq1or Bo1
107. Spbio_001264
108. Spbio_002028
109. Methocarbamol [usp-rs]
110. Methocarbamol [who-dd]
111. Bpbio1_000119
112. Gtpl6829
113. Methocarbamol (jan/usp/inn)
114. (+-)-3-(o-methoxyphenoxy)-1,2-propanediol 1-carbamate
115. Chembl1201117
116. Dtxsid6023286
117. Hms501f16
118. Kbio1_000434
119. Kbio2_001477
120. Kbio2_004045
121. Kbio2_006613
122. Kbio3_001491
123. Ninds_000434
124. Hms1568f09
125. Hms1920l19
126. Hms2091d14
127. Hms2095f09
128. Hms2233g18
129. Hms3259m17
130. Hms3369b17
131. Hms3655o06
132. Hms3712f09
133. Methocarbamol [green Book]
134. Pharmakon1600-01500397
135. Methocarbamol [orange Book]
136. Bcp13801
137. Hy-b0262
138. Tox21_110454
139. Ccg-39655
140. Methocarbamol [usp Monograph]
141. Nsc170960
142. Nsc757112
143. S1736
144. Akos015960743
145. Tox21_110454_1
146. Db00423
147. Nc00509
148. Nsc-757112
149. Idi1_000434
150. Robaxisal Component Methocarbamol
151. Ncgc00018257-02
152. Ncgc00018257-03
153. Ncgc00018257-04
154. Ncgc00018257-07
155. Ncgc00018257-08
156. Ncgc00018257-16
157. Ncgc00089805-02
158. Ncgc00089805-03
159. 1, 3-(o-methoxyphenoxy)-, 1-carbamate
160. Ac-12037
161. As-11691
162. 1, 3-(2-methoxyphenoxy)-, 1-carbamate
163. Methocarbamol Component Of Robaxisal
164. Sbi-0051442.p003
165. 3-o-(2-methoxyphenyl)-1-glyceryl Carbamate
166. Ab00052040
167. Ft-0628302
168. Ft-0671053
169. M2254
170. Sw196781-3
171. D00402
172. D70323
173. Ab00052040-15
174. Ab00052040_16
175. Ab00052040_17
176. 2-hydroxy-3-(2-methoxyphenoxy)propyl Carbamate #
177. A829442
178. Q411456
179. Sr-01000000240-2
180. Sr-01000000240-3
181. (rs)-2-hydroxy-3-(2-methoxyphenoxy)propyl Carbamate
182. Brd-a31521121-001-05-8
183. Guaiacol Glyceryl Ether Carbamate, Analytical Standard
184. (+/-)-3-( O-methoxyphenoxy)-1,2-propanediol 1-carbamate
185. Carbamic Acid 2-hydroxy-3-(2-methoxyphenoxy)propyl Ester
186. Carbamic Acid [2-hydroxy-3-(2-methoxy-phenoxy)-propyl] Ester
187. (+/-)-3-(o-methoxyphenoxy)-1,2-propanediol 1-carbamate
188. Methocarbamol, United States Pharmacopeia (usp) Reference Standard
189. 1,2-propanediol, 3-(2-methoxyphenoxy)-, 1-carbamate, (+/-)-
190. Methocarbamol, Pharmaceutical Secondary Standard; Certified Reference Material
191. 3967-43-9
| Molecular Weight | 241.24 g/mol |
|---|---|
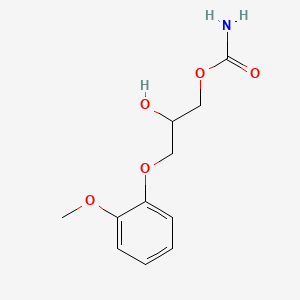
| Molecular Formula | C11H15NO5 |
| XLogP3 | 0.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 7 |
| Exact Mass | 241.09502258 g/mol |
| Monoisotopic Mass | 241.09502258 g/mol |
| Topological Polar Surface Area | 91 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 236 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Methocarbamol |
| PubMed Health | Methocarbamol |
| Drug Classes | Skeletal Muscle Relaxant, Centrally Acting |
| Drug Label | robaxin/robaxin-750 (methocarbamol tablets, USP), a carbamate derivative of guaifenesin, is a central nervous system (CNS) depressant with sedative and musculoskeletal relaxant properties.The chemical name of methocarbamol is 3-(2-methoxyphenoxy)... |
| Active Ingredient | Methocarbamol |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 500mg; 750mg |
| Market Status | Prescription |
| Company | Hikma Intl Pharms; Vintage Pharms; Hetero Labs Ltd Iii; Sandoz; Prinston; Lannett Holdings; Watson Labs; Austarpharma |
| 2 of 6 | |
|---|---|
| Drug Name | Robaxin |
| PubMed Health | Methocarbamol |
| Drug Classes | Skeletal Muscle Relaxant, Centrally Acting |
| Drug Label | robaxin/robaxin-750 (methocarbamol tablets, USP), a carbamate derivative of guaifenesin, is a central nervous system (CNS) depressant with sedative and musculoskeletal relaxant properties.The chemical name of methocarbamol is 3-(2-methoxyphenoxy)... |
| Active Ingredient | Methocarbamol |
| Dosage Form | Tablet; Injectable |
| Route | Injection; Oral |
| Strength | 500mg; 100mg/ml |
| Market Status | Prescription |
| Company | Auxilium Pharms; Hikma Maple |
| 3 of 6 | |
|---|---|
| Drug Name | Robaxin-750 |
| PubMed Health | Methocarbamol (By mouth) |
| Drug Classes | Skeletal Muscle Relaxant, Centrally Acting |
| Active Ingredient | Methocarbamol |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 750mg |
| Market Status | Prescription |
| Company | Auxilium Pharms |
| 4 of 6 | |
|---|---|
| Drug Name | Methocarbamol |
| PubMed Health | Methocarbamol |
| Drug Classes | Skeletal Muscle Relaxant, Centrally Acting |
| Drug Label | robaxin/robaxin-750 (methocarbamol tablets, USP), a carbamate derivative of guaifenesin, is a central nervous system (CNS) depressant with sedative and musculoskeletal relaxant properties.The chemical name of methocarbamol is 3-(2-methoxyphenoxy)... |
| Active Ingredient | Methocarbamol |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 500mg; 750mg |
| Market Status | Prescription |
| Company | Hikma Intl Pharms; Vintage Pharms; Hetero Labs Ltd Iii; Sandoz; Prinston; Lannett Holdings; Watson Labs; Austarpharma |
| 5 of 6 | |
|---|---|
| Drug Name | Robaxin |
| PubMed Health | Methocarbamol |
| Drug Classes | Skeletal Muscle Relaxant, Centrally Acting |
| Drug Label | robaxin/robaxin-750 (methocarbamol tablets, USP), a carbamate derivative of guaifenesin, is a central nervous system (CNS) depressant with sedative and musculoskeletal relaxant properties.The chemical name of methocarbamol is 3-(2-methoxyphenoxy)... |
| Active Ingredient | Methocarbamol |
| Dosage Form | Tablet; Injectable |
| Route | Injection; Oral |
| Strength | 500mg; 100mg/ml |
| Market Status | Prescription |
| Company | Auxilium Pharms; Hikma Maple |
| 6 of 6 | |
|---|---|
| Drug Name | Robaxin-750 |
| PubMed Health | Methocarbamol (By mouth) |
| Drug Classes | Skeletal Muscle Relaxant, Centrally Acting |
| Active Ingredient | Methocarbamol |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 750mg |
| Market Status | Prescription |
| Company | Auxilium Pharms |
Muscle Relaxants, Central
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Skeletal muscle relaxants are indicated as adjunts to other measures, such as rest and physical therapy, for the relief of muscle spasm associated with acute, painful musculoskeletal conditions. /Included in US product label/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2625
Methcarbamol is also FDA-approved for control of the neuromuscular manifestations of tetanus. However it has largely been replaced in the treatment of tetanus by diazepam, or, in severe cases a neuromuscular blocking agent such as pancuronium. Such therapy is used as an adjunct to other measures, such as debridement, tetanus antitoxin, penicillin, tracheotomy, fluid and electrolyte replacement, and supportive treatment.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2625
VET: In dogs, cats, and horses, methocarbamol is indicated as adjunct therapy of acute inflammatory and traumatic conditions of skeletal muscle and to reduce muscle spasms.
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2017
For more Therapeutic Uses (Complete) data for METHOCARBAMOL (6 total), please visit the HSDB record page.
The most frequent adverse effects of methocarbamol are drowsiness, dizziness, and lightheadedness. Blurred vision, headache, fever, and nausea may occur after oral, IM, or IV administration of the drug. Anorexia has been reported after oral administration. Adynamic ileus occurred in one patient who received a total of 10 g of methocarbamol orally. Metallic taste, GI upset, nystagmus, diplopia, flushing, vertigo, mild muscular incoordination, syncope, hypotension, and bradycardia have occurred in patients receiving the drug IM or IV.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007.
Allergic reactions such as urticaria, pruritus, rash, skin eruptions, and conjunctivitis with nasal congestion may occur in patients receiving methocarbamol. Anaphylactic reactions have occurred following IM or IV administration of the drug. Although most patients with methocarbamol-induced syncope recover with supportive treatment, epinephrine, corticosteroids, and/or antihistamines have been used to increase the rate of recovery in some of these patients.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007.
When methocarbamol is administered IV, thrombophlebitis, sloughing, and pain at the injection site may result from extravasation. IM injection of the drug may also cause local irritation. IV injection of methocarbamol may cause a small amount of hemolysis and increased hemoglobin and red blood cells in the urine. Leukopenia may occur rarely.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007.
Parenteral dosage forms should be used with caution in patients with epilepsy.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2629
For more Drug Warnings (Complete) data for METHOCARBAMOL (14 total), please visit the HSDB record page.
Methocarbamol tablets and intramuscular injections are indicated in the United States as an adjunct to rest, physical therapy, and other measures for the relief of discomforts associated with acute, painful musculoskeletal conditions. Oral methocarbamol in America may be given up to 1500mg 4 times daily for 2-3 days. In Canada, methocarbamol containing oral formulations are sold over the counter for pain associated with muscle spasm. However, if these combination formulations include codeine, they are prescription only.
FDA Label
Methacarbamol is a skeletal muscle relaxant with an unknown mechanism of action. Methacarbamol has been shown to block spinal polysynaptic reflexes, decrease nerve transmission in spinal and supraspinal polysynaptic pathways, and prolong the refractory period of muscle cells. Methocarbamol does not act as a local anesthetic upon injection. In animal studies, methocarbamol also prevents convulsions after electric shock.
Muscle Relaxants, Central
A heterogeneous group of drugs used to produce muscle relaxation, excepting the neuromuscular blocking agents. They have their primary clinical and therapeutic uses in the treatment of muscle spasm and immobility associated with strains, sprains, and injuries of the back and, to a lesser degree, injuries to the neck. They have been used also for the treatment of a variety of clinical conditions that have in common only the presence of skeletal muscle hyperactivity, for example, the muscle spasms that can occur in MULTIPLE SCLEROSIS. (From Smith and Reynard, Textbook of Pharmacology, 1991, p358) (See all compounds classified as Muscle Relaxants, Central.)
M - Musculo-skeletal system
M03 - Muscle relaxants
M03B - Muscle relaxants, centrally acting agents
M03BA - Carbamic acid esters
M03BA03 - Methocarbamol
Absorption
The time to maximum concentration is 1.1 hours for both healthy patients and those on hemodialysis. The maximum plasma concentration is 21.3mg/L for healthy patients and 28.7mg/L in hemodialysis patients. The area under the curve for healthy patients is 52.5mg/L\*hr and 87.1mg/L*hr in hemodialysis patients. AUC% based on terminal elimination half life is 2% for healthy patients and 4% for hemodialysis patients. Older studies report maximum plasma concentrations in 0.5 hours.
Route of Elimination
In humans the majority of the dose is eliminated in the urine. In dogs, 88.85% of the dose is eliminated in urine and 2.14% in the feces. In rats, 84.5-92.5% of the dose is eliminated in the urine and 0-13.3% is eliminated in the feces.
Volume of Distribution
Volume of distribution data in humans is scarce. In horses, the volume of distribution is 515-942mL/kg at steady state or 724-1130mL/kg.
Clearance
0.2-0.8L/h/kg.
Methocarbamol is rapidly and almost completely absorbed from the GI tract. Blood or serum concentrations of methocarbamol required for sedative, skeletal muscle relaxant, or toxic effects are not known.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007.
Following oral administration of a single dose of methocarbamol, peak blood or serum concentrations of the drug appear to be attained in approximately 1-2 hours; the onset of action is usually within 30 minutes. Data from an unpublished study indicate that peak blood concentrations (measured as total carbamates and expressed in terms of methocarbamol) average 16.5 mcg/mL following a single 2-g oral dose, while data from a published study (using an assay relatively specific for methocarbamol) indicate that peak serum concentrations average 29.8 mcg/mL following the same dose. Data from the unpublished study also indicate that after IV administration of 1 g of methocarbamol at a rate of 300 mg/minute, blood concentrations of 19 mcg/mL are attained immediately and that the onset of action is almost immediate.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007.
In dogs, methocarbamol is widely distributed, with highest concentrations attained in the kidney and liver; lower concentrations are attained in the lungs, brain, and spleen, and low concentrations are attained in heart and skeletal muscle.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007.
The drug and/or its metabolites cross the placenta in dogs. It is not known if methocarbamol is distributed into milk in humans.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007.
For more Absorption, Distribution and Excretion (Complete) data for METHOCARBAMOL (8 total), please visit the HSDB record page.
Methocarbamol is metabolized in the liver by demethylation to 3-(2-hydroxyphenoxy)-1,2-propanediol-1-carbamate or hydroxylation to 3-(4-hydroxy-2-methoxyphenoxy)-1,2-propanediol-1-carbamate. Methocarbamol and its metabolites are conjugated through glucuronidation or sulfation.
Methocarbamol is extensively metabolized, presumably in the liver, by dealkylation and hydroxylation.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007.
Based on limited data, about 10-15% of a single oral dose is excreted in urine as unchanged drug, about 40-50% as the glucuronide and sulfate conjugates of 3-(2-hydroxyphenoxy)-1,2-propanediol-1-carbamate and 3-(4-hydroxy-2-methoxyphenoxy)-1,2-propanediol-1-carbamate, and the remainder as unidentified metabolites.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007.
In dogs, rats and in man, methocarbamol gave p-hydroxymethocarbamol and o-demethylation product. All three substances were excreted in urine as glucuronic acid and ester sulfate conjugates.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 206
Permethylation and g.l.c.-mass spectrometric analysis of bile from an isolated rat liver perfusion to which methocarmol was added showed seven components not present in control bile: methocarbamol, glucuronides of methocarbamol and desmethyl-methocarbamol, and four glucuronides of hydroxylated methocarbamol metabolites. 2. An interesting rearrangement of a methyl group has been found in the mass spectrum of 3-(2-methoxyphenyloxy)-1,2-dimethoxypropane, the permethylation product from methocarbamol.
PMID:1166662 Thompson RM, Gerber N et al; Xenobiotica 5 (3): 145-53 (1975)
The elimination half life is 1.14 hours in healthy subjects and 1.24 hours in subjects with renal insufficiency. Older studies report half lives of 1.6-2.15 hours.
Methocarbamol has a serum half-life of 0.9-1.8 hours.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007.
... Pharmacokinetics of methocarbamol were studied in eight healthy, adult horses after intravenous (iv) and oral administration of large dosages. ... Plasma methocarbamol concentration declined very rapidly during the initial or rapid disposition phase after iv administration; the terminal elimination half-life ranged from 59 to 90 mins. ...
PMID:9109959 Muir W, Sams R et al; Equine Vet J Suppl (11): 41-4 (1992)
/Investigators/ determined plasma methocarbamol concentrations over 24 hr following a 1.5 g methocarbamol dose (off-dialysis day) to 8 chronic hemodialysis patients and compared these results to those from 17 healthy male volunteers. The harmonic mean elimination half-life was similar between the two groups, 1.24 and 1.14 hr, respectively. ...
PMID:2253675 Sica D, Comstock T et al; Eur J Clin Pharmacol 39 (2): 193-4 (1990)
... Pharmacokinetics of methocarbamol were studied in eight healthy, adult horses after intravenous (iv) and oral administration of large dosages. ... Plasma methocarbamol concentration declined very rapidly during the initial or rapid disposition phase after iv administration; the terminal elimination half-life ranged from 59 to 90 mins. ...
PMID:9109959 Muir W, Sams R et al; Equine Vet J Suppl (11): 41-4 (1992)
The mechanism of action of methocarbamol is thought to be dependant on its central nervous system depressant activity. This action may be mediated through blocking spinal polysynaptic reflexes, decreasing nerve transmission in spinal and supraspinal polysynaptic pathways, and prolonging the refractory period of muscle cells. Methocarbamol has been found to have no effect on contraction of muscle fibres, motor end plates, or nerve fibres.
Precise mechanism of action has not been determined. These agents act in the central nervous system (CNS) rather than directly on skeletal muscle. Several of these medications have been shown to depress polysynaptic reflexes preferentially. The muscle relaxant effects of most of these agents may be related to their CNS depressant (sedative) effects. /Skeletal Muscle Relaxants/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2625