


1. Brevimytal Natrium
2. Brevital
3. Brietal
4. Brietal Sodium
5. Brietal-sodium
6. Methohexital Sodium
7. Methohexital, Monosodium Salt
8. Methohexitone
9. Monosodium Salt Methohexital
10. Natrium, Brevimytal
11. Sodium, Methohexital
1. Methohexitone
2. 151-83-7
3. Methodrexitone
4. Enallynymall
5. Methohexitalum
6. Metohexital
7. Brietal
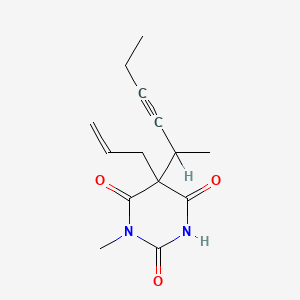
8. 5-allyl-5-(3-hexyn-2-yl)-1-methylbarbituric Acid
9. Compound 22451
10. Compound 25398
11. 18652-93-2
12. (+-)-5-allyl-1-methyl-5-(1-methyl-2-pentynyl)barbituric Acid
13. 5-hex-3-yn-2-yl-1-methyl-5-prop-2-enyl-1,3-diazinane-2,4,6-trione
14. Chebi:102216
15. 5-allyl-1-methyl-5-(1-methyl-2-pentynyl)-2,4,6(1h,3h,5h)-pyrimidinetrione
16. E5b8nd5ipe
17. Metoesital
18. 5-allyl-1-methyl-5-(1-methyl-pent-2-ynyl)-pyrimidine-2,4,6-trione
19. Enallynymalum
20. Barbituric Acid, 5-allyl-1-methyl-5-(1-methyl-2-pentynyl)-
21. Alpha-dl-1-methyl-5-allyl-5-(1'-methylpentyn-2-yl)barbituric Acid
22. Metoesital [dcit]
23. (+/-)-5-allyl-1-methyl-5-(1-methyl-2-pentynyl)barbituric Acid
24. .alpha.-dl-1-methyl-5-allyl-5-(1'-methylpentyn-2-yl)barbituric Acid
25. Barbituric Acid, 5-allyl-1-methyl-5-(1-methyl-2-pentynyl)-, (.+/-.)-
26. Metohexital [inn-spanish]
27. Dea No. 2264
28. Methohexitalum [inn-latin]
29. Methohexital (usp/inn)
30. Unii-e5b8nd5ipe
31. Methohexital [usp:inn:ban]
32. Methohexital Civ
33. Einecs 205-798-6
34. Methohexital [mi]
35. Methohexital [inn]
36. Epitope Id:117125
37. Chembl7413
38. Methohexital [vandf]
39. Schembl80729
40. Methohexital [mart.]
41. Methohexital [who-dd]
42. Gtpl7233
43. Dtxsid1023287
44. Methohexital Civ [usp-rs]
45. Act02624
46. Methohexital [usp Monograph]
47. Methohexital 1.0 Mg/ml In Methanol
48. 5-allyl-5-(hex-3-yn-2-yl)-1-methylpyrimidine-2,4,6(1h,3h,5h)-trione
49. Db00474
50. 1-methyl-5-(1-methylpent-2-yn-1-yl)-5-(prop-2-en-1-yl)pyrimidine-2,4,6(1h,3h,5h)-trione
51. 1-methyl-5-(1-methylpent-2-yn-1-yl)-5-prop-2-en-1-ylpyrimidine-2,4,6(1h,3h,5h)-trione
52. 2,4,6(1h,3h,5h)-pyrimidinetrione, 1-methyl-5-(1-methyl-2-pentynyl)-5-(2-propenyl)-
53. 5-(hex-3-yn-2-yl)-1-methyl-5-(prop-2-en-1-yl)-1,3-diazinane-2,4,6-trione
54. 2,4,6(1h,3h,5h)-pyrimidinetrione, 1-methyl-5-(1-methyl-2-pentynyl)-5-(2-propenyl)-, (+/-)-
55. Bm164664
56. Db-043117
57. C07844
58. D04985
59. A809228
60. Q851813
61. 1-methyl-5-(1-methyl-2-pentynyl)-5-allyl-barbituric Acid
62. Dl-1-methyl-5-(1-methyl-2-pentynyl)-5-(2-propenyl)-2,4,6(1h,3h,5h)-pyrimidinetrione
63. 2,4(3h,5h)-pyrimidinedione, 6-hydroxy-3-methyl-5-(1-methyl-2-pentyn-1-yl)-5-(2-propen-1-yl)-
64. 2,4,6(1h,3h,5h)-pyrimidinetrione, 1-methyl-5-(1-methyl-2-pentynyl)-5-(2-propenyl)-, (+-)-
65. 2,4,6(1h,3h,5h)-pyrimidinetrione, 1-methyl-5-(1-methyl-2-pentynyl)-5-(2-propenyl)-, (.+/-.)-
66. 5-(hex-3-yn-2-yl)-6-hydroxy-3-methyl-5-(prop-2-en-1-yl)-2,3,4,5-tetrahydropyrimidine-2,4-dione
| Molecular Weight | 262.30 g/mol |
|---|---|
| Molecular Formula | C14H18N2O3 |
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 262.13174244 g/mol |
| Monoisotopic Mass | 262.13174244 g/mol |
| Topological Polar Surface Area | 66.5 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 497 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Methohexital is indicated for use as an intravenous anaesthetic. It has also been commonly used to induce deep sedation.
FDA Label
Methohexital, a barbiturate, is used for the induction of anesthesia prior to the use of other general anesthetic agents and for induction of anesthesia for short surgical, diagnostic, or therapeutic procedures associated with minimal painful stimuli. Little analgesia is conferred by barbiturates; their use in the presence of pain may result in excitation.
Anesthetics, Intravenous
Ultrashort-acting anesthetics that are used for induction. Loss of consciousness is rapid and induction is pleasant, but there is no muscle relaxation and reflexes frequently are not reduced adequately. Repeated administration results in accumulation and prolongs the recovery time. Since these agents have little if any analgesic activity, they are seldom used alone except in brief minor procedures. (From AMA Drug Evaluations Annual, 1994, p174) (See all compounds classified as Anesthetics, Intravenous.)
N - Nervous system
N01 - Anesthetics
N01A - Anesthetics, general
N01AF - Barbiturates, plain
N01AF01 - Methohexital
N - Nervous system
N05 - Psycholeptics
N05C - Hypnotics and sedatives
N05CA - Barbiturates, plain
N05CA15 - Methohexital
Absorption
The absolute bioavailability following rectal administration of methohexital is 17%.
Route of Elimination
Excretion occurs via the kidneys through glomerular filtration.
Metabolism occurs in the liver through demethylation and oxidation. Side-chain oxidation is the most important biotransformation involved in termination of biologic activity.
5.6 ± 2.7 minutes
Methohexital binds at a distinct binding site associated with a Cl- ionopore at the GABAA receptor, increasing the duration of time for which the Cl- ionopore is open. The post-synaptic inhibitory effect of GABA in the thalamus is, therefore, prolonged.