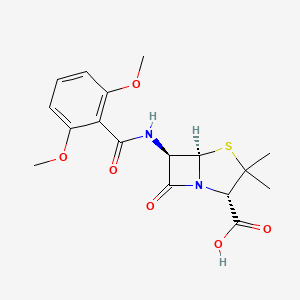

1. Dimethoxyphenyl Penicillin
2. Methicillin Hydrate, Monosodium Salt
3. Methicillin Monohydrate, Monosodium Salt
4. Methicillin Sodium
5. Meticillin
6. Metin
7. Penicillin, Dimethoxyphenyl
8. Staphcillin
1. Meticillin
2. Methycillin
3. 61-32-5
4. Methicillinum
5. Meticilina
6. Meticilline
7. Meticillinum
8. Staphcillin
9. Metacillin
10. Dimocillin
11. Meticilina [inn-spanish]
12. Meticilline [inn-french]
13. Meticillinum [inn-latin]
14. (2,6-dimethoxyphenyl)penicillin
15. 6-(2,6-dimethoxybenzamido)penicillanic Acid
16. Chebi:6827
17. (2s,5r,6r)-6-[(2,6-dimethoxybenzoyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid
18. Brl 1241
19. 6beta-(2,6-dimethoxybenzamido)penicillanic Acid
20. 2,6-dimethoxyphenyl Penicillin
21. Meticillina
22. Q91fh1328a
23. Celbenin
24. Meticillina [dcit]
25. (2s,5r,6r)-6-(2,6-dimethoxybenzamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid
26. Meticillin [inn]
27. Methicillin [usan]
28. Hsdb 3121
29. Meticillin [inn:ban]
30. Einecs 200-505-8
31. Penicillin, (2,6-dimethoxyphenyl)-
32. Methcilline
33. Unii-q91fh1328a
34. (2s,5r,6r)-6-(2,6-dimethoxybenzamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid
35. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 6-((2,6-dimethoxybenzoyl)amino)-3,3-dimethyl-7-oxo-, (2s-(2alpha,5alpha,6beta))-
36. Spectrum_000993
37. Spectrum2_001965
38. Spectrum3_000494
39. Spectrum4_000878
40. Spectrum5_001600
41. Meticillin [hsdb]
42. Chembl575
43. Epitope Id:139649
44. Methicillin [vandf]
45. Schembl4898
46. Meticillin [who-dd]
47. Bspbio_001987
48. Kbiogr_001575
49. Kbioss_001473
50. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 6-(2,6-dimethoxybenzamido)-3,3-dimethyl-7-oxo-
51. 6-(2,3-dimethoxybenzamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid
52. Divk1c_000100
53. Spbio_002089
54. Dtxsid6023284
55. Kbio1_000100
56. Kbio2_001473
57. Kbio2_004041
58. Kbio2_006609
59. Kbio3_001487
60. Ninds_000100
61. Zinc3831070
62. Bdbm50103523
63. 6beta-(2,6-dimethoxybenzamido)-2,2-dimethylpenam-3alpha-carboxylic Acid
64. Db01603
65. Idi1_000100
66. (2s,5r,6r)-6-{[(2,6-dimethoxyphenyl)carbonyl]amino}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid
67. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 6-(2,6-dimethoxybenzamido)-3,3,-dimethyl-7-oxo-
68. Mii
69. Sbi-0051440.p003
70. Hy-121544
71. Mrsa Selective Supplement, For Microbiology
72. Cs-0082724
73. C07177
74. Q409262
75. Brd-k34388247-236-02-5
76. (2s,5r,6r)-6-[(2,6-dimethoxybenzene)amido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid
| Molecular Weight | 380.4 g/mol |
|---|---|
| Molecular Formula | C17H20N2O6S |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 5 |
| Exact Mass | 380.10420754 g/mol |
| Monoisotopic Mass | 380.10420754 g/mol |
| Topological Polar Surface Area | 131 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 600 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Penicillins
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Methicillin /is/ indicated in the treatment of bone and joint infections caused by susceptible organisms. /Included in US product labeling; Not included in Canadian product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 2149
Methicillin /is/ indicated in the treatment of bacterial endocarditis caused by susceptible organisms. /Included in US product labeling; Not included in Canadian product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 2149
Methicillin /is/ indicated in the treatment of bacterial septicemia caused by susceptible organisms. /Included in US product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 2150
For more Therapeutic Uses (Complete) data for METHICILLIN (7 total), please visit the HSDB record page.
METHICILLIN MUST NOT BE USED IN PREFERENCE TO PENICILLIN G IN INFECTIONS AMENDABLE TO TREATMENT WITH LATTER DRUG.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1075
IMPORTANT BIOLOGICAL EFFECT OF PENICILLIN, UNRELATED TO HYPERSENSITIVITY OR TO "TOXIC" REACTION, IS ALTERATION OF BACTERIAL FLORA IN AREAS OF BODY TO WHICH IT GAINS ACCESS. /PENICILLIN/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1150
IM INJECTION OF METHICILLIN IS MORE PAINFUL THAN IS CASE FOR OTHER PENICILLINS; NEED FOR FREQUENT INJECTIONS (EVERY 2-3 HR) IS DEFINITE DISADVANTAGE WHEN PROLONGED THERAPY BY THIS ROUTE IS REQUIRED.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1143
IN SOME PERSONS...SUPRAINFECTION RESULTS FROM CHANGES IN FLORA. DERMATITIS INVOLVING PRIMARILY SCROTAL & INGUINAL SKIN...HAS BEEN OBSERVED... DRAMATIC EFFECT THAT MAY FOLLOW USE OF PENICILLIN IN SYPHILIS IS JARISCH-HERXHEIMER REACTION... /PENICILLINS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1150
For more Drug Warnings (Complete) data for METHICILLIN (21 total), please visit the HSDB record page.
Used to treat infections caused by susceptible Gram-positive bacteria, particularly beta-lactamase-producing organisms such as Staphylococcus aureus that would otherwise be resistant to most penicillins.
Meticillin (INN, BAN) or methicillin (USAN) is a narrow spectrum beta-lactam antibiotic of the penicillin class. It is no longer clinically used. Its role in therapy has been largely replaced by flucloxacillin and dicloxacillin, however the term methicillin-resistant Staphylococcus aureus (MRSA) continues to be used to describe Staphylococcus aureus strains resistant to all penicillins.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01C - Beta-lactam antibacterials, penicillins
J01CF - Beta-lactamase resistant penicillins
J01CF03 - Meticillin
Absorption
Not absorbed following oral administration.
METHICILLIN IS NOT EMPLOYED BY ORAL ROUTE BECAUSE IT IS POORLY ABSORBED & READILY DESTROYED BY ACIDIC GASTRIC CONTENTS. WHEN DRUG IS GIVEN IM, PEAK PLASMA CONCN ARE REACHED IN ABOUT 30 MIN-1 HR. AFTER CONVENTIONAL DOSE OF 1 G IN ADULTS, PLASMA CONCN IN EXCESS OF 10 UG/ML ARE DEMONSTRABLE...
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1075
...2-G DOSE PROVIDES PEAK CONCN OVER 20 UG/ML, & APPROX 8 UG/ML IS STILL PRESENT AFTER 4 HR. ABOUT 40% OF METHICILLIN IN PLASMA IS BOUND TO PROTEIN. METHICILLIN DOES NOT READILY PENETRATE INTO CSF; HOWEVER, SIGNIFICANT CONCN ARE PRESENT IN PATIENTS WITH MENINGITIS... DRUG...WELL DISTRIBUTED IN VARIOUS BODY FLUIDS & TISSUES.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1075
METHICILLIN IS EXCRETED UNCHANGED IN URINE...ABOUT 2/3 OF IM DOSE IS ELIMINATED BY THIS ROUTE IN 4 HR. ... METHICILLIN PERSISTS FOR LONG PERIOD & @ HIGH CONCN IN PLASMA IN CASES OF RENAL FAILURE. ... PORTION OF INJECTED METHICILLIN THAT CANNOT BE DETECTED IN URINE IS EXCRETED INTO BILE & IS ELIMINATED BY WAY OF FECES.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1143
DRUGS RECENTLY SHOWN TO ACTIVELY CROSS HUMAN PLACENTA INCL...SODIUM METHICILLIN... /METHICILLIN SODIUM/
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 433
For more Absorption, Distribution and Excretion (Complete) data for METHICILLIN (21 total), please visit the HSDB record page.
Hepatic (20-40%).
YIELDS 2,6-DIMETHOXYPHENYLPENICILLOIC ACID IN BACILLUS. /FROM TABLE/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. D-80
25-60 minutes
The serum half-life of methicillin in adults with normal renal function is 0.4-0.5 hours.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 95. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1995 (Plus Supplements 1995)., p. 268
Serum concn of methicillin may be higher and the serum half-life prolonged in patients with impaired renal function. The serum half-life of the drug reportedly averages 4-6 hr in anuric patients.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 95. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1995 (Plus Supplements 1995)., p. 268
In one study in children 2-16 yr of age, the serum half-life of methicillin averaged 0.8 hr after IV administration and 1.6 hr after IM administration. Serum concn of methicillin are generally higher and the serum half-life is longer in neonates than in older children. The serum half-life of the drug is generally inversely proportional to birthweight, gestational age, and chronologic age. The serum half-life of methicillin reportedly ranges from 2-3.9 hr in low birthweight neonates 2 wk of age or younger and ranges from 0.9-3.3 hr in low birthweight neonates older than 2 wk of age or neonates weighing 2 kg or more who are 6.5 wk of age or younger.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 95. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1995 (Plus Supplements 1995)., p. 268
Similar to other beta-lactam antimicrobials, meticillin blocks synthesis of the bacterial cell wall. Meticillin stops cross-linkage between the peptidoglycan polymer chains, which make up a large portion of gram-positive bacterial cell walls. It does this by binding to and competitively inhibiting the transpeptidase enzyme used by bacteria to cross-link the peptide (D-alanyl-alanine) used in peptidogylcan synthesis.
The penicillins and their metabolites are potent immunogens because of their ability to combine with proteins and act as haptens for acute antibody-mediated reactions. The most frequent (about 95 percent) or "major" determinant of penicillin allergy is the penicilloyl determinant produced by opening the beta-lactam ring of the penicillin. This allows linkage of the penicillin to protein at the amide group. "Minor" determinants (less frequent) are the other metabolites formed, including native penicillin and penicilloic acids. /Penicillins/
Haddad, L.M., Clinical Management of Poisoning and Drug Overdose. 2nd ed. Philadelphia, PA: W.B. Saunders Co., 1990., p. 953
Bactericidal; inhibit bacterial cell wall synthesis. Action is dependent on the ability of penicillins to reach and bind penicillin-binding proteins (PBPs) located on the inner membrane of the bacterial cell wall. Penicillin-binding proteins (which include transpeptidases, carboxypeptidases, and endopeptidases) are enzymes that are involved in the terminal stages of assembling the bacterial cell wall and in reshaping the cell wall during growth and division. Penicillins bind to, and inactivate, penicillin-binding proteins, resulting in the weakening of the bacterial cell wall and lysis. /Penicillins/
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 2150