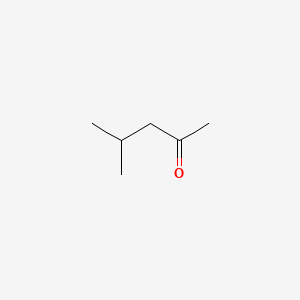

1. 4-methyl-2-pentanone
2. Isobutyl Methyl Ketone
3. Isopropylacetone
1. 4-methyl-2-pentanone
2. 4-methylpentan-2-one
3. 108-10-1
4. Isopropylacetone
5. Isobutyl Methyl Ketone
6. Mibk
7. 2-pentanone, 4-methyl-
8. Hexone
9. 2-methyl-4-pentanone
10. 4-methyl-2-oxopentane
11. Methylisobutylketon
12. Isohexanone
13. Hexon
14. Shell Mibk
15. Metilisobutilchetone
16. Metyloizobutyloketon
17. 2-methylpropyl Methyl Ketone
18. Isobutyl-methylketon
19. Methyl-isobutyl-cetone
20. 4-methyl-2-pentanon
21. 4-metilpentan-2-one
22. Isopropyl Acetone
23. Ketone, Isobutyl Methyl
24. 4-methyl-pentan-2-on
25. 4-methyl-pentan-2-one
26. Rcra Waste Number U161
27. Fema No. 2731
28. Nsc 5712
29. Methyl Isobutylketone
30. Methyl Iso-butyl Ketone
31. 2-methyl-4-pentanal
32. Methyl I-butyl Ketone
33. Ethyl Iso-butyl Ketone
34. Mfcd00008938
35. U5t7b88cnp
36. Methyl Isobutyl Ketone [nf]
37. Chebi:82344
38. Nsc-5712
39. Hexon [czech]
40. Methyl Isobutyl Ketone (nf)
41. Mik
42. Methylisobutyl Ketone
43. 4-methyl-2-pentanone, >=99%
44. Caswell No. 574aa
45. Fema Number 2731
46. Methylisobutylketone
47. Isobutyl-methylketon [czech]
48. Metyloizobutyloketon [polish]
49. Isobutylmethyl Ketone
50. Metilisobutilchetone [italian]
51. 4-methyl-2-pentanon [czech]
52. Ccris 2052
53. Hsdb 148
54. Methyl-isobutyl-cetone [french]
55. 4-metilpentan-2-one [italian]
56. 4-methyl-2-pentanone (natural)
57. 2-pentanone,4-methyl-
58. Methylisobutylketon [dutch, German]
59. Einecs 203-550-1
60. Un1245
61. Rcra Waste No. U161
62. Unii-u5t7b88cnp
63. Epa Pesticide Chemical Code 044105
64. Brn 0605399
65. 4-methyl-pentan-2-on [dutch, German]
66. Ai3-01229
67. Methylisobutyketone
68. Isobutylmethylketone
69. Methylisobutlyketone
70. I-bucome
71. Methylisobutyl Keton
72. Methylisobutyl-keton
73. Methylpentan-2-one
74. Iso-butylmethylketone
75. Methyl-isobutylketone
76. 4-methyl-2pentanone
77. Methy Isobutyl Ketone
78. Methyl Isobutyl Keton
79. Methyl Iso-butylketone
80. Methyl-iso-butylketone
81. Methyl-isobutyl Ketone
82. 4-methylpentane-2-one
83. Iso-c4h9coch3
84. Methyl-2-pentanon,4-
85. 4-methyl- 2-pentanone
86. Mibk [inci]
87. Dsstox_cid_1889
88. Ec 203-550-1
89. Dsstox_rid_76387
90. Dsstox_gsid_21889
91. Schembl15458
92. Isopropylacetone [mi]
93. 4-01-00-03305 (beilstein Handbook Reference)
94. 4-methyl-2-pentanone(mibk)
95. Chembl285323
96. Dtxsid5021889
97. Schembl13341539
98. Nsc5712
99. Methyl Isobutyl Ketone, Acs Grade
100. Amy11098
101. Zinc1482107
102. 4-methyl-2-pentanone, Hplc Grade
103. Methylisobutylketon(dutch, German)
104. Tox21_201108
105. Wln: 1y1 & 1v1
106. 4-methyl-2-pentanone [fcc]
107. Lmfa12000033
108. Methylisobutylketone [usp-rs]
109. Methyl Isobutyl Ketone [hsdb]
110. Methyl Isobutyl Ketone [iarc]
111. 4-methyl-2-pentanone [fhfi]
112. Akos000118793
113. 4-methyl-2-pentanone, Ar, >=99%
114. 4-methyl-2-pentanone, Lr, >=99%
115. Methyl Isobutyl Ketone [mart.]
116. Un 1245
117. 4-methyl-2-pentanone, >=99%, Fcc
118. Methyl Isobutyl Ketone [usp-rs]
119. Ncgc00091475-01
120. Ncgc00091475-02
121. Ncgc00258660-01
122. 4-methyl-pentan-2-on(dutch, German)
123. Bp-13453
124. Cas-108-10-1
125. Methyl Isobutyl Ketone Reagent Grade Acs
126. 4-methyl-2-pentanone, Analytical Standard
127. Ft-0628744
128. M0389
129. 4-methyl-2-pentanone, Technical Grade, 95%
130. 4-methyl-2-pentanone, For Hplc, >=99.5%
131. C19263
132. D04989
133. 4-methyl-2-pentanone, Acs Reagent, >=98.5%
134. A801806
135. Q418104
136. 4-methyl-2-pentanone, Saj First Grade, >=99.0%
137. J-515799
138. Methyl Isobutyl Ketone, P.a., Acs Reagent, 98.5%
139. Q-200495
140. 2-pentanone,4-methyl Methyl,isobutyl,ketone
141. 4-methyl-2-pentanone, Jis Special Grade, >=99.5%
142. Methyl Isobutyl Ketone [un1245] [flammable Liquid]
143. F1908-0087
144. 4-methyl-2-pentanone 1000 Microg/ml In N,n-dimethylformamide
145. 4-methyl-2-pentanone, Puriss. P.a., Acs Reagent, >=99.0% (gc)
146. 4-methyl-2-pentanone, Puriss., Acs Reagent, Reag. Ph. Eur., 99.0%
147. 4-methyl-2-pentanone, Suitable For Atomic Absorption Spectrometry, >=99.5%
148. Alfa-[(phenylmethoxy)carbonyl]oxy-1-piperidineaceticacidmethylester
149. Methyl Isobutyl Ketone, United States Pharmacopeia (usp) Reference Standard
150. Methyl Isobutyl Ketone, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 100.16 g/mol |
|---|---|
| Molecular Formula | C6H12O |
| XLogP3 | 1.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 2 |
| Exact Mass | 100.088815002 g/mol |
| Monoisotopic Mass | 100.088815002 g/mol |
| Topological Polar Surface Area | 17.1 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 64.6 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Methyl isobutyl ketone (MIBK) is a solvent used in numerous products and processes and may be present in the air of the workplace as a vapor. The American Conference of Governmental Industrial Hygienists (ACGIH) threshold limit value-time-weighted average (TLV-TWA) and TLV-short term exposure limit (TLV-STEL) for MIBK are 50 and 75 ppm, respectively. These workplace air concentration limits were set to protect workers from irritation, neurasthenic symptoms and possible adverse effects to their livers and kidneys. A recent revision of the ACGIH limit value has been proposed, to reduce the current TLV-TWA to 30 ppm. This article predicts the kinetics and accumulation of MIBK in humans exposed repeatedly in various exposure scenarios (8, 12, and 24 hr/day for 7 days) to the current ACGIH TLV-TWA of 50 ppm. The kinetic parameters of the model were derived from published human time-course blood MIBK data from a single 2 hr inhalation exposure to 48.9 ppm MIBK. The model correctly simulated single exposure experimental data with a rapid rise in blood concentration to 1.06 ug/mL within 1 hr and approached >or=99% steady-state blood level in 4 hr of exposure. MIBK was predicted to be rapidly eliminated from blood after terminating the exposure, reaching 0.53 ug/mL and 0.13 ug/mL within 0.5 and 2 hr post-exposure, respectively. Within 4h after the termination of exposure, blood concentration would be expected to <1% of the steady-state concentration. On the basis of these results, it is concluded that accumulation of MIBK in workers due to repeated inhalation exposure is not likely to occur at the current TLV-TWA concentration of 50 ppm.
PMID:18789368 Saghir SA, Rick DL; Regul Toxicol Pharmacol 52 (2): 180-8 (2008)
Methyl isobutyl ketone /was reported/ in human maternal blood samples collected immediately after delivery, indicating the potential for the compound to enter the umbilical cord and cross the placenta.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V101 312-3 (2013)
Methyl isobutyl ketone was detected in the brain, liver, lung, vitreous fluid, kidney, and blood in two workers who died after exposure to several organic solvents during spray painting. One died from a fall and the other died of cerebral edema 9 hours later. Tissue concentrations (mg/100 g) were reported to be: case 1 - brain, 0.25; liver, 0.49; lung, 0.43; vitreous fluid, 0.52; kidney, 0.24; and femoral blood, 0.14; and case 2 - brain, 0.06; liver, 0.22; lung, 0.11; vitreous fluid, 0.02; kidney, 0.08; and heart blood, 0.04.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V101 312 (2013)
Human volunteers (98 men and women) were exposed to 100 ppm (410 mg/cu m) methyl isobutyl ketone for 4 hours in an environmental chamber. Steady-state blood concentrations of methyl isobutyl ketone were attained after 2 hours of exposure. Blood and breath samples collected 90 minutes after exposure indicated that most of the absorbed compound had been eliminated from the body.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V101 312 (2013)
For more Absorption, Distribution and Excretion (Complete) data for Methyl isobutyl ketone (11 total), please visit the HSDB record page.
Methyl isobutyl ketone (MIBK) has recently been shown to potentiate the cholestasis induced by manganese-bilirubin (Mn-BR) or manganese (Mn) in rats. In this study, we investigated the effect of 4-methyl-2-pentanol (4MPOL) and 4-hydroxymethyl isobutyl ketone (4-OHMIBK), two major metabolites of MIBK, on these models of cholestasis. Dosages varying from 1.88 to 15 mmol/kg 4MPOL or 4-OHMIBK were administered once, 18 hr prior to the administration of a cholestatic Mn-BR combination. The cholestatic effect of the manganese-bilirubin combination was enhanced with dosages of 4MPOL of 3.75 mmol/kg and larger. On the other hand, dosages smaller than 15 mmol/kg of 4-OHMIBK did not potentiate the Mn-BR cholestasis. Daily pretreatment for 3 days resulted in an increased response to the cholestatic challenges of either Mn-BR or Mn alone. 4MPOL administered repetitively was a better potentiator than 4-OHMIBK with the Mn-BR model of cholestasis. However, with Mn alone, 4-OHMIBK proved to be more effective. 4MPOL and 4-OHMIBK per se were devoid of cholestatic properties, since the bile flow measured prior to the cholestatic challenge was not decreased and in some cases was significantly greater than that of vehicle-pretreated animals. These results show that MIBK metabolites can potentiate the cholestatic form of hepatotoxicity. /4-methyl-2-pentanol and 4-hydroxymethyl isobutyl ketone (metabolites)/
PMID:3353988 Vezina M, Plaa GL; Toxicol Appl Pharmacol 92 (3): 419-27 (1988)
The absorption and metabolism of MIBK was studied in male Sprague-Dawley rats orally administered a single dose of 5 mmol/kg body weight of MIBK in corn oil, equivalent to 501 mg/kg body weight, by gavage. MIBK was rapidly absorbed into the systemic circulation following oral exposure, with a mean maximum plasma concentration (Cmax) of 0.644 mmol/L occurring at 0.25 hours [(time to maximum plasma concentration (tmax)] post-administration. MIBK was detected at very low levels (0.006 mmol/L) at 9 hours post-administration. The plasma levels of methyl isobutyl carbinol (MIBC) were very low (<0.012 mmol/L) all over the study. The major material in the blood was 4-hydroxymethyl-4-methyl-2-pentanone (referred to as HMP based on the chemical name) [i.e., diacetone alcohol (DAA], with a Cmax of 2.03 mmol/L at 9 hours and remained detectable at 12 hours post-dosing. Neither MIBK nor MIBC were detectable in 12-hour samples. No compounds other than HMP and MIBK were detected in the blood. The 12-hour area under the plasma concentration time curve (AUC0-12 h) for MIBK, MIBC and HMP were 0.089, 3.558 and 17, 436 mmol/L/hour, respectively. HMP and MIBK represented 79% and 20% of the total AUC, respectively. The plasma elimination half-life (t1/2) of MIBK and HMP were 2.529 and 4.831 hours, respectively. Based on the results of this study, MIBK is rapidly absorbed into the blood in rats following oral exposure and is rapidly and extensively metabolized to HMP (the major metabolite in blood after 3 hours).
European Chemicals Agency (ECHA); Registered Substances, 4-Methylpentan-2-one (CAS Number: 108-10-1) (EC Number: 203-550-1) (Last modified: January 22, 2018). Available from, as of February 21, 2018: https://echa.europa.eu/
Methyl isobutyl carbinol (MIBC) is an oxygenated solvent that is metabolized to methylisobutyl ketone (MIBK) and then to 4-hydroxymethyl-4-methyl-2-pentanone (HMP). Plasma levels of MIBC, MIBK and HMP were determined up to 12 hr after a single oral 5 mmol/kg dose of MIBC or MIBK to male rats. The major material in the plasma in both cases was HMP, with similar areas-under-the-curve (AUC) and C(max) at 9 hr after dosing. MIBK plasma levels and AUC were also comparable after MIBK or MIBC administration. MIBC AUC was only about 6% of the total material in the blood after MIBC, and insignificant after MIBK administration. No other metabolites were detected in the plasma under the analytical conditions used. The extent of metabolism of MIBC to MIBK, by comparing combined AUCs for MIBK and HMP, was at least 73%. ...
PMID:12505273 Gingell R et al; Toxicol Lett 136 (3): 199-204 (2003)
The metabolic fate of methyl n-butyl ketone (MnBK) and its isomer methyl isobutyl ketone (MiBK) was studied in mice. The concentrations of both ketones and their metabolites in blood and brain were measured at different time intervals after their administration. The principal metabolites of ... MiBK were 4-methyl-2-pentanol (4-MPOL) and 4-hydroxy-4 methyl-2-pentanone (HMP). .... The administration of 4-methyl-2-pentanol resulted in the appearance of MiBK and HMP. The administration of HMP did not result in the appearance of MiBK or 4-MPOL. ....
PMID:8284793 Granvil CP et al; Toxicol Lett 70 (3): 263-7 (1994)
For more Metabolism/Metabolites (Complete) data for Methyl isobutyl ketone (7 total), please visit the HSDB record page.
Blood: initial fast phase = 12 minutes, then slow phase = 1 hour; [TDR, p. 886]
TDR - Ryan RP, Terry CE, Leffingwell SS (eds). Toxicology Desk Reference: The Toxic Exposure and Medical Monitoring Index, 5th Ed. Washington DC: Taylor & Francis, 1999., p. 886
The absorption and metabolism of MIBK was studied in male Sprague-Dawley rats orally administered a single dose of 5 mmol/kg body weight of MIBK in corn oil, equivalent to 501 mg/kg body weight, by gavage. ... The plasma elimination half-life (t1/2) of MIBK ... was 2.529 hours ... .
European Chemicals Agency (ECHA); Registered Substances, 4-Methylpentan-2-one (CAS Number: 108-10-1) (EC Number: 203-550-1) (Last modified: January 22, 2018). Available from, as of February 21, 2018: https://echa.europa.eu/
In Guinea pigs serum half-lives and clearance times for methyl isobutyl ketone was 66 min and 6 hr respectively.
PMID:941151 Divincenzo GD et al; Toxicol Appl Pharmacol 36 (3): 511-22 (1976)