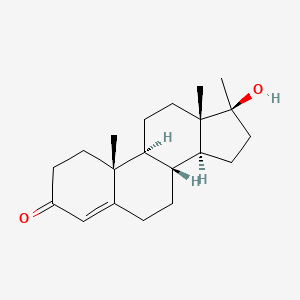

1. 17 Alpha Methyltestosterone
2. 17 Beta Hydroxy 17 Methyl 4 Androsten 3 One
3. 17 Beta Methyltestosterone
4. 17 Beta-hydroxy-17-methyl-4-androsten-3-one
5. 17 Beta-methyltestosterone
6. 17 Epimethyltestosterone
7. 17-alpha-methyltestosterone
8. 17-epimethyltestosterone
9. 17alpha Methyl Testosterone
10. 17alpha Methyltestosterone
11. 17alpha-methyl-testosterone
12. 17alpha-methyltestosterone
13. 17beta Hydroxy 17 Methyl 4 Androsten 3 One
14. 17beta Methyltestosterone
15. 17beta-hydroxy-17-methyl-4-androsten-3-one
16. 17beta-methyltestosterone
17. Android (methyltestoterone)
18. Android 10
19. Android 25
20. Android 5
21. Android-10
22. Android-25
23. Android-5
24. Mesteron
25. Mesterone
26. Metandren
27. Methitest
28. Oreton
29. Testoviron
30. Testred
31. Virilon
1. 17-methyltestosterone
2. Testred
3. Metandren
4. Android
5. Virilon
6. 58-18-4
7. Androsan
8. Androsten
9. Mesterone
10. 17alpha-methyltestosterone
11. Malestrone
12. Mastestona
13. Synandrets
14. Synandrotabs
15. Testhormone
16. Andrometh
17. Anertan
18. Dumogran
19. Homandren
20. Hormale
21. Malogen
22. Masenone
23. Metestone
24. Metrone
25. Nabolin
26. Oraviron
27. Steronyl
28. Syndren
29. Testora
30. Oreton Methyl
31. Glosso-sterandryl
32. Oreton-m
33. M.t.mucorettes
34. Neo-hombreol-m
35. Nu-man
36. Android 5
37. Android 10
38. Android 25
39. Methitest
40. Nsc-9701
41. Orchisterone-m
42. Testovis Depot
43. Homandren, Tablets
44. Metiltestosterona
45. Methyltestosteronum
46. 17beta-hydroxy-17-methylandrost-4-en-3-one
47. Ru 24400
48. 17-methyltestosteron
49. Cdb 110
50. Anertan, Tablets
51. 17-hydroxy-17-methyl-3-keto-androstene-4
52. 17alpha-methyl-3-oxo-4-androsten-17beta-ol
53. 17-beta-hydroxy-17-methylandrost-4-en-3-one
54. 17.alpha.-methyltestosterone
55. 4-androstene-17alpha-methyl-17beta-ol-3-one
56. Methyltestosterone Ciii
57. L 589.372
58. 17
59. A-methyltestosterone
60. Methyl Testosterone
61. U 2842
62. V9efu16zif
63. 17alpha-methyl-delta-androsten-17beta-ol-3-one
64. Nsc-139965
65. Chebi:27436
66. Oreton M
67. Androsan, Tablets
68. Cdb-110
69. Component Of Estan
70. (17beta)-17-hydroxy-17-methylandrost-4-en-3-one
71. Neo-hombreol [m]
72. Neo-homobreol (m)
73. Neo-homobreol [m]
74. Component Of Gynetone
75. (8r,9s,10r,13s,14s,17s)-17-hydroxy-10,13,17-trimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1h-cyclopenta[a]phenanthren-3-one
76. Androst-4-en-3-one, 17-hydroxy-17-methyl-, (17b)-
77. Ncgc00091009-03
78. Androst-4-en-3-one, 17-hydroxy-17-methyl-, (17.beta.)-
79. Component Of Tylosterone
80. Estratest
81. Ru-24400
82. Glosso Sterandryl
83. Testosterone, 17-methyl-
84. U-2842
85. Dsstox_cid_13664
86. Dsstox_rid_79089
87. Anertan (tablets)
88. Dsstox_gsid_33664
89. Homandren (van)
90. Testovis (tablet)
91. 17.alpha.-methyl-3-oxo-4-androsten-17.beta.-ol
92. Androsan (tablets)
93. Androsan (van)
94. Testoviron (van)
95. Anertan (van)
96. 4-androstene-17.alpha.-methyl-17.beta.-ol-3-one
97. Androst-4-en-3-one, 17.beta.-hydroxy-17-methyl-
98. L-589372
99. Testoviron (tablet)
100. L-589.372
101. Oretron
102. Metiltestosterone
103. Estan (salt/mix)
104. Metiltestosterone [dcit]
105. 17-alpha-methyltestosterone
106. Cas-58-18-4
107. Estratest (salt/mix)
108. Smr000058528
109. Android (tn)
110. Ccris 3723
111. Methyltestosteronum [inn-latin]
112. Metiltestosterona [inn-spanish]
113. Hsdb 3365
114. Wln: L E5 B666 Ov Mutj A E Fq F -b&aef
115. Einecs 200-366-3
116. Unii-v9efu16zif
117. Nsc 139965
118. Brn 2057425
119. Component Of Gynetone (salt/mix)
120. Premarin With Methyltestosterone
121. 4-androstene-17-alpha-methyl-17-beta-ol-3-one
122. Ncgc00091009-04
123. Ncgc00091009-05
124. Androst-4-en-3-one, 17beta-hydroxy-17-methyl-
125. Testred (tn)
126. (17-beta)-17-hydroxy-17-methylandrost-4-en-3-one
127. Premarin With Methyltestosterone (salt/mix)
128. 17(alpha)-methyl-delta4-androsten-17(beta)-ol-3-one
129. 17-hydroxy-17-methylandrost-4-en-3-one #
130. 17a-methyltestosterone
131. Methyltestosterone [usp:inn:ban:jan]
132. 17-.beta.-hydroxy-17-methylandrost-4-en-3-one
133. Androst-4-en-3-on-17.beta.-ol, 17.alpha.-methyl
134. Androst-4-ene-17.alpha.-methyl-17.beta.-ol-3-one
135. 17.beta.-hydroxy-17.alpha.-methylandrost-4-en-3-one
136. 17.alpha.-methyl-.delta.4-androsten-17.beta.-ol-3-one
137. Chembl1395
138. Schembl18657
139. 4-08-00-01010 (beilstein Handbook Reference)
140. Androst-4-en-3-one, 17-beta-hydroxy-17-methyl-
141. Mls000759474
142. Mls001424040
143. Mls002174282
144. Gtpl6945
145. Methyltestosterone [inn]
146. Methyltestosterone [jan]
147. Dtxsid1033664
148. Methyltestosterone [hsdb]
149. .alpha.-methyltestosterone
150. Methyltestosterone [vandf]
151. Nsc9701
152. 17-mt
153. Hms2051a14
154. Hms2272a06
155. Methyltestosterone [mart.]
156. Methyltestosterone [usp-rs]
157. Methyltestosterone [who-dd]
158. Methyltestosterone [who-ip]
159. 17-methyltestosterone [mi]
160. Hy-a0121
161. Zinc3814422
162. Tox21_113161
163. Tox21_113162
164. Tox21_400058
165. Bdbm50410531
166. Lmst02020029
167. Methyltestosterone (jp17/usp/inn)
168. Nsc139965
169. Akos015917317
170. Tox21_113162_1
171. Ccg-100871
172. Cs-5099
173. Db06710
174. Gs-6594
175. Nc00121
176. Methyltestosterone [orange Book]
177. Methyltestosterone [ep Monograph]
178. Ncgc00091009-01
179. Ncgc00091009-06
180. Ncgc00091009-07
181. Oxandrolone Impurity, Methyltestosterone-
182. (8r,9s,10r,13s,14s,17s)-17-hydroxy-10,13,17-trimethyl-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3h-cyclopenta[a]phenanthren-3-one
183. Methyltestosterone [usp Monograph]
184. Methyltestosteronum [who-ip Latin]
185. Smr001261452
186. M0435
187. 17alpha-methyltestosterone, >=97.0% (hplc)
188. C07198
189. D00408
190. Methyltestosterone (17alpha-methyltestosterone)
191. Ab00443683-06
192. Ab00443683-09
193. 17alpha-methyl-delta4-androsten-17beta-ol-3-one
194. 17alpha-methyltestosterone, Solid (photosensitive)
195. Q421768
196. 17alpha-methyl-delta(4)-androsten-17beta-ol-3-one
197. 17-alpha-methyltestosterone 100 Microg/ml In Methanol
198. 17.beta.-hydroxy-17-methylandrost-4-en-3-one
199. 17(alpha)-methyl-delta(4)-androsten-17(beta)-ol-3-one
200. 17-alpha-methyltestosterone 100 Microg/ml In Acetonitrile
201. 17alpha-methyltestosterone, Vetranal(tm), Analytical Standard
202. Oxandrolone Impurity, Methyltestosterone- [usp Impurity]
203. Methyltestosterone, European Pharmacopoeia (ep) Reference Standard
204. Methyltestosterone (17alpha-methyltestosterone) 1.0 Mg/ml In Acetonitrile
205. Methyltestosterone, United States Pharmacopeia (usp) Reference Standard
206. Methyltestosterone For System Suitability, European Pharmacopoeia (ep) Reference Standard
207. (1s,2r,10r,11s,14s,15s)-14-hydroxy-2,14,15-trimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one
208. (8r,10r,13s,17s)-17-hydroxy-10,13,17-trimethyl-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-cyclopenta[a]phenanthren-3-one
209. 17alpha-methyltestosterone Solution, 1.0 Mg/ml In 1,2-dimethoxyethane, Ampule Of 1 Ml, Certified Reference Material
210. Methyltestosterone Aka ''(8r,9s,10r,13s,14s,17s)-17-hydroxy-10,13,17-trimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1h-cyclopenta[a]phenanthren-3-one
| Molecular Weight | 302.5 g/mol |
|---|---|
| Molecular Formula | C20H30O2 |
| XLogP3 | 3.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 302.224580195 g/mol |
| Monoisotopic Mass | 302.224580195 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 550 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 8 | |
|---|---|
| Drug Name | Android 10 |
| PubMed Health | Methyltestosterone (By mouth) |
| Drug Classes | Endocrine-Metabolic Agent |
| Drug Label | The androgens are steroids that develop and maintain primary and secondary male sex characteristics.Androgens are derivatives of cyclopentanoperhydrophenanthrene. Endogenous androgens are C-19 steroids with a side chain at C-17, and with two angular... |
| Active Ingredient | Methyltestosterone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 10mg |
| Market Status | Prescription |
| Company | Valeant Pharm Intl |
| 2 of 8 | |
|---|---|
| Drug Name | Android 25 |
| PubMed Health | Methyltestosterone (By mouth) |
| Drug Classes | Endocrine-Metabolic Agent |
| Drug Label | The androgens are steroids that develop and maintain primary and secondary male sex characteristics.Androgens are derivatives of cyclopentanoperhydrophenanthrene. Endogenous androgens are C-19 steroids with a side chain at C-17, and with two angular... |
| Active Ingredient | Methyltestosterone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg |
| Market Status | Prescription |
| Company | Valeant Pharm Intl |
| 3 of 8 | |
|---|---|
| Drug Name | Methyltestosterone |
| Active Ingredient | Methyltestosterone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 10mg |
| Market Status | Prescription |
| Company | Impax Labs |
| 4 of 8 | |
|---|---|
| Drug Name | Testred |
| Active Ingredient | Methyltestosterone |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 10mg |
| Market Status | Prescription |
| Company | Valeant Pharm Intl |
| 5 of 8 | |
|---|---|
| Drug Name | Android 10 |
| PubMed Health | Methyltestosterone (By mouth) |
| Drug Classes | Endocrine-Metabolic Agent |
| Drug Label | The androgens are steroids that develop and maintain primary and secondary male sex characteristics.Androgens are derivatives of cyclopentanoperhydrophenanthrene. Endogenous androgens are C-19 steroids with a side chain at C-17, and with two angular... |
| Active Ingredient | Methyltestosterone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 10mg |
| Market Status | Prescription |
| Company | Valeant Pharm Intl |
| 6 of 8 | |
|---|---|
| Drug Name | Android 25 |
| PubMed Health | Methyltestosterone (By mouth) |
| Drug Classes | Endocrine-Metabolic Agent |
| Drug Label | The androgens are steroids that develop and maintain primary and secondary male sex characteristics.Androgens are derivatives of cyclopentanoperhydrophenanthrene. Endogenous androgens are C-19 steroids with a side chain at C-17, and with two angular... |
| Active Ingredient | Methyltestosterone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg |
| Market Status | Prescription |
| Company | Valeant Pharm Intl |
| 7 of 8 | |
|---|---|
| Drug Name | Methyltestosterone |
| Active Ingredient | Methyltestosterone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 10mg |
| Market Status | Prescription |
| Company | Impax Labs |
| 8 of 8 | |
|---|---|
| Drug Name | Testred |
| Active Ingredient | Methyltestosterone |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 10mg |
| Market Status | Prescription |
| Company | Valeant Pharm Intl |
Anabolic Steroids; Androgens, Synthetic; Antineoplastic Agents, Hormonal
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Androgens are primarily indicated in males as replacement therapy when congenital or acquired endogenous androgen absence or deficiency is associated with primary hypogonadal or secondary hypogonadism. Primary hypogonadism includes conditions such as testicular failure due to cryptorchidism, bilateral torsion, orchitis, or vanishing testis syndrome; inborn errors in testosterone biosynthesis; or bilateral orchidectomy. Hypogonadotropic hypogonadism (secondary hypogonadism) conditions include gonadotropin releasing hormone (GnRH) deficiency; or pituitary hypothalamic injury as a result of surgery, tumors, trauma, or radiation and are the most common forms of hypogonadism seen in older adults. Dosage adjustment is needed to accommodate individual clinical requirements for such life changes as induction of puberty, development of secondary sexual characteristics, impotence due to testicular failure, or infertility due to oligospermia.. /Androgens; Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 150
A 6 month or shorter course of an androgen is indicated for induction of puberty in patients with familial delayed puberty, a condition characterized by spontaneous, nonpathologic, late-onset puberty, if the patient does not respond to psychological treatment. /Androgens; Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 151
Androgens are used in the treatment of constitutional delay in growth. However, they are not longer considered the treatment of choice for most patients. /Androgens; NOT included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 151
For more Therapeutic Uses (Complete) data for 17-METHYLTESTOSTERONE (6 total), please visit the HSDB record page.
Cholestatic hepatitis and jaundice and abnormal liver function test results may occur in patients receiving 17-alpha-alkylandrogens such as methyltestosterone. These adverse hepatic effects may occur at relatively low doses of the drug. Drug-induced jaundice usually is reversible following discontinuance of the drug. Methyltestosterone should be discontinued if cholestatic jaundice or hepatitis occurs, or if liver function test results become abnormal during therapy with the drug, and the etiology of these disorders should be determined. Peliosis of the liver and hepatic neoplasms, including hepatocellular carcinoma, have been reported rarely in patients receiving long-term administration of androgenic anabolic steroids. Peliosis of the liver can be a life-threatening or fatal complication of androgen therapy.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 2948
It is not known whether methyltestosterone is distributed into milk. Because of the potential for serious adverse reactions to androgens in nursing infants, a decision should be made whether to discontinue nursing or to not use methyltestosterone, taking into account the importance of the drug to the woman.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 2948
Methyltestosterone is contraindicated in males with carcinoma of the breast or known or suspected carcinoma of the prostate. Some manufacturers state that the drug also is contraindicated in patients with cardiac, renal, or hepatic decompensation; hypercalcemia; impaired liver function; and in patients who are easily sexually stimulated. Because of the potential risk of serious adverse health effects, methyltestosterone should not be used for enhancement of athletic performance or physique.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 2948
Since the risks clearly outweigh the possible benefits in women who are or may become pregnant, methyltestosterone is contraindicated in such women. Women who become pregnant while receiving the drug should be informed of the potential hazard to the fetus.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 2948
For more Drug Warnings (Complete) data for 17-METHYLTESTOSTERONE (20 total), please visit the HSDB record page.
Methyltestosterone is an anabolic steroid hormone used to treat men with a testosterone deficiency. It is also used in women to treat breast cancer, breast pain, swelling due to pregnancy, and with the addition of estrogen it can treat symptoms of menopause.
Testosterone is a steroid hormone from the androgen group. Testosterone is primarily secreted from the testes of males. In females, it is produced in the ovaries, adrenal glands and by conversion of adrostenedione in the periphery. It is the principal male sex hormone and an anabolic steroid. In both males and females, it plays key roles in health and well-being. Examples include enhanced libido, energy, immune function, and protection against osteoporosis. On average, the adult male body produces about twenty times the amount of testosterone than an adult female's body does.
Anabolic Agents
These compounds stimulate anabolism and inhibit catabolism. They stimulate the development of muscle mass, strength, and power. (See all compounds classified as Anabolic Agents.)
Antineoplastic Agents, Hormonal
Antineoplastic agents that are used to treat hormone-sensitive tumors. Hormone-sensitive tumors may be hormone-dependent, hormone-responsive, or both. A hormone-dependent tumor regresses on removal of the hormonal stimulus, by surgery or pharmacological block. Hormone-responsive tumors may regress when pharmacologic amounts of hormones are administered regardless of whether previous signs of hormone sensitivity were observed. The major hormone-responsive cancers include carcinomas of the breast, prostate, and endometrium; lymphomas; and certain leukemias. (From AMA Drug Evaluations Annual 1994, p2079) (See all compounds classified as Antineoplastic Agents, Hormonal.)
G - Genito urinary system and sex hormones
G03 - Sex hormones and modulators of the genital system
G03B - Androgens
G03BA - 3-oxoandrosten (4) derivatives
G03BA02 - Methyltestosterone
G - Genito urinary system and sex hormones
G03 - Sex hormones and modulators of the genital system
G03E - Androgens and female sex hormones in combination
G03EK - Androgens and female sex hormones in combination with other drugs
G03EK01 - Methyltestosterone
Absorption
The methyl group aids to increase oral bioavailability.
Route of Elimination
90% urine / 10% feces
Absorbed from oral mucosa and gastrointestinal tract.
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 151
Sublingual methyltestosterone is absorbed faster and its bioavailability is double that from orally admin drug in humans...
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 166
It is not known whether methyltestosterone is distributed into milk.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 2948
Hepatic. Testosterone is metabolized to 17-keto steroids through two different pathways. The major active metabolites are estradiol and dihydrotestosterone (DHT).
17alpha-methyl-5beta-androstan-3alpha,16beta,17beta-triol...its 16-epimer 17alpha-methyl-5beta-androstan-3alpha,16alpha,17beta-triol...and 3alpha,17beta-dihydroxy-17alpha-methyl-5beta-androstan-16-one...have been identified as urinary metabolites of /17alpha-methyltestosterone/ of treated rabbits.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 230
6-8 hours
2.5 to 3.5 hours
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 152
The effects of testosterone in humans and other vertebrates occur by way of two main mechanisms: by activation of the androgen receptor (directly or as DHT), and by conversion to estradiol and activation of certain estrogen receptors. Free testosterone (T) is transported into the cytoplasm of target tissue cells, where it can bind to the androgen receptor, or can be reduced to 5α-dihydrotestosterone (DHT) by the cytoplasmic enzyme 5α-reductase. DHT binds to the same androgen receptor even more strongly than T, so that its androgenic potency is about 2.5 times that of T. The T-receptor or DHT-receptor complex undergoes a structural change that allows it to move into the cell nucleus and bind directly to specific nucleotide sequences of the chromosomal DNA. The areas of binding are called hormone response elements (HREs), and influence transcriptional activity of certain genes, producing the androgen effects.