


1. Bay F 1353
2. Bay-f 1353
3. Bayf 1353
4. Baypen
5. Melocin
6. Meslocillin
7. Mezlin
8. Mezlocillin
9. Mezlocillin Sodium
10. Mezlocilline
11. Sodium, Mezlocillin
1. 80495-46-1
2. Mezlocillin Sodium Monohydrate [orange Book]
3. 3cww8b5904
4. Baycipen
5. Baypen
6. Mezlocillin Sodium (jan)
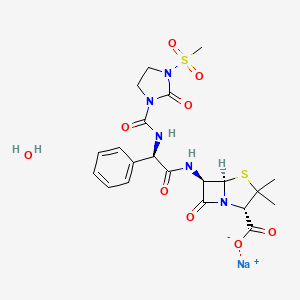
7. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 3,3-dimethyl-6-(((2r)-(((3-(methylsulfonyl)-2-oxo-1-imidazolidinyl)carbonyl)amino)phenylacetyl)amino)-7-oxo-, Monosodium Salt, Monohydrate, (2s,5r,6r)-
8. Sodium (2s,5r,6r)-3,3-dimethyl-6-((r)-2-(3-(methylsulfonyl)-2-oxo-1-imidazolidinecarboxamido)-2-phenylacetamido)-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylate, Monohydrate
9. Mezlocillin Sodium [jan]
10. Unii-3cww8b5904
11. Mezlin (tn)
12. Schembl1237585
13. Chebi:52068
14. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 3,3-dimethyl-6-(((((3-(methylsulfonyl)-2-oxo-1-imidazolidinyl)carbonyl)amino)phenylacetyl)amino)-7-oxo-, Monosodium Salt, Monohydrate (2s-(2alpha,5alpha,6beta(s*)))-
15. Mezlocillin Sodium Salt Monohydrate
16. D02221
17. Mezlocillin Sodium Monohydrate [who-dd]
18. Mezlocillin Sodium Salt Monohydrate [mi]
19. Q27123146
20. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 3,3-dimethyl-6-(((((3-(methylsulfonyl)-2-oxo-1-imidazolidinyl)carbonyl)amino)phenylacetyl)amino)-7-oxo-, Monosodium Salt, Monohydrate (2s-(2.alpha.,5.alpha.,6.beta.(s*)))-
21. Sodium (2s,5r,6r)-3,3-dimethyl-6-{[(2r)-2-({[3-(methylsulfonyl)-2-oxoimidazolidin-1-yl]carbonyl}amino)-2-phenylacetyl]amino}-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate Hydrate
22. Sodium 6beta-{(2r)-2-[3-(methanesulfonyl)-2-oxoimidazolidine-1-carboxamido]-2-phenylacetamido}-2,2-dimethylpenam-3alpha-carboxylate--water (1/1)
23. Sodium 6beta-{(2r)-2-[3-(methanesulfonyl)-2-oxoimidazolidine-1-carboxamido]-2-phenylacetamido}penicillanate Hydrate
| Molecular Weight | 579.6 g/mol |
|---|---|
| Molecular Formula | C21H26N5NaO9S2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 6 |
| Exact Mass | 579.10696405 g/mol |
| Monoisotopic Mass | 579.10696405 g/mol |
| Topological Polar Surface Area | 211 Ų |
| Heavy Atom Count | 38 |
| Formal Charge | 0 |
| Complexity | 1090 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)