
1. Brentan
2. Dactarin
3. Miconasil Nitrate
4. Miconazole Nitrate
5. Monistat
6. Nitrate, Miconasil
7. Nitrate, Miconazole
8. R 14,889
9. R-14,889
10. R14,889
1. 22916-47-8
2. Monistat
3. Monistat Iv
4. Daktarin Iv
5. Miconazol
6. Miconazolo
7. Miconazolum
8. Minostate
9. Monistat-derm
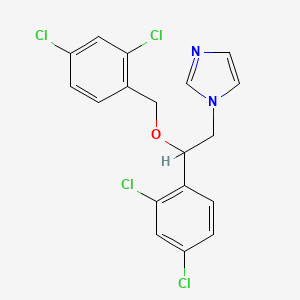
10. 1-[2-(2,4-dichlorophenyl)-2-[(2,4-dichlorophenyl)methoxy]ethyl]-1h-imidazole
11. Mjr 1762
12. Miconazolum [inn-latin]
13. Vusion
14. Florid(nitrate)
15. 1-(2-((2,4-dichlorobenzyl)oxy)-2-(2,4-dichlorophenyl)ethyl)-1h-imidazole
16. Brentan
17. R 18134
18. 1-[2-(2,4-dichlorophenyl)-2-[(2,4-dichlorophenyl)methoxy]ethyl]imidazole
19. Miconazole 3
20. Nsc 170986
21. Daktarin
22. Miconazole (monistat)
23. Miconazole 7
24. Monistat 3
25. Monistat 5
26. Monistat 7
27. Monistat 1 Combination Pack
28. Chembl91
29. Monistat (tn)
30. Mfcd00216019
31. Miconazole 7 Combination Pack
32. 1-(2,4-dichloro-beta-((2,4-dichlorobenzyl)oxy)phenethyl)imidazole
33. 7nno0d7s5m
34. R18134 Nitrate
35. 1h-imidazole, 1-(2-(2,4-dichlorophenyl)-2-((2,4-dichlorophenyl)methoxy)ethyl)-
36. 1h-imidazole, 1-[2-(2,4-dichlorophenyl)-2-[(2,4-dichlorophenyl)methoxy]ethyl]-
37. Mcz
38. Dactarin
39. Chebi:82892
40. Monistat 3 Combination Pack
41. 22916-47-8 (free)
42. Nsc-170986
43. Miconazolo [dcit]
44. Ncgc00016770-01
45. Micozole
46. Zimycan
47. 1-(2,4-dichloro-beta-((2,4-dichlorobenzyl)oxy)phenethyl) Imidazole
48. Imidazole, 1-(2,4-dichloro-beta-((2,4-dichlorobenzyl)oxy)phenethyl)-
49. Femizol-m
50. Miconazole-7
51. Imidazole, 1-(2-(2,4-dichlorophenyl)-2-((2,4-dichlorophenyl)methoxy)ethyl)-
52. Monazole 7
53. 1-[2-(2,4-dichlorophenyl)-2-{[(2,4-dichlorophenyl)methyl]oxy}ethyl]-1h-imidazole
54. Miconazol [inn-spanish]
55. Oravig
56. Aflorix(nitrate)
57. 1-[2-(2,4-dichlorobenzyloxy)-2-(2,4-dichlorophenyl)ethyl]-1h-imidazole
58. 1-{2-[(2,4-dichlorobenzyl)oxy]-2-(2,4-dichlorophenyl)ethyl}-1h-imidazole
59. Imidazole, 1-(2,4-dichloro-beta-((2,4-dichlorobenzyl)oxy) Phenethyl)-
60. Albistat(nitrate)
61. Andergin(nitrate)
62. Conofite(nitrate)
63. (+/-)-miconazole Nitrate Salt
64. 1h-imidazole, 1-(2-(2,4-dichlorophenyl)-2-((2,4-dichlorophenyl) Methoxy)ethyl)-
65. Imidazole, 1-(2-(2,4-dichlorophenyl)-2-((2,4-dichlorophenyl) Methoxy)ethyl)- (9ci)
66. Monista (nitrate)
67. Micantin (nitrate)
68. Novo-miconazole Vaginal Ovules
69. Ccris 7924
70. Gyno-daktar(nitrate)
71. Lotrimin Af(nitrate)
72. Nsc169434
73. Epi-monistat(nitrate)
74. Monistat 7 Vaginal Suppositories
75. Miconazole Nitrate Salt
76. Einecs 245-324-5
77. Unii-7nno0d7s5m
78. Brn 0965511
79. Zimybase
80. Miconazole Base
81. 1-(2,4-dichloro-beta-[(2,4-dichlorobenzyl)oxy]phenethyl)imidazole
82. Sr-01000000271
83. 1-(2-(2,4-dichlorobenzyloxy)-2-(2,4-dichlorophenyl)ethyl)-1h-imidazole
84. 1-[2-[(2,4-dichlorobenzyl)oxy]-2-(2,4-dichlorophenyl)ethyl]-1h-imidazole
85. 1-(2-(2,4-dichlorophenyl)-2-((2,4-dichlorophenyl)methoxy)ethyl)-1h-imidazole
86. Miconazole [usp:inn:ban:jan]
87. Prestwick_335
88. Oravig (tn)
89. Spectrum_000965
90. Miconazole [mi]
91. Miconazole [inn]
92. Miconazole [jan]
93. Prestwick0_000067
94. Prestwick1_000067
95. Prestwick2_000067
96. Prestwick3_000067
97. Spectrum2_001048
98. Spectrum3_000507
99. Spectrum4_000061
100. Spectrum5_001297
101. Dsstox_cid_3319
102. Miconazole [vandf]
103. Bmse000924
104. (+-)-1-(2,4-dichloro-beta-((2,4-dichlorobenzyl)oxy)phenethyl)imidazole
105. Cid_4189
106. Miconazole [mart.]
107. Schembl2866
108. Dsstox_rid_76975
109. Miconazole [who-dd]
110. Dsstox_gsid_23319
111. Oprea1_091955
112. Bspbio_000253
113. Bspbio_002033
114. Kbiogr_000581
115. Kbioss_001445
116. Mls002222203
117. Divk1c_000156
118. Spbio_000976
119. Spbio_002174
120. Bpbio1_000279
121. Gtpl2449
122. Miconazole (jp17/usp/inn)
123. Dtxsid6023319
124. Miconazole [ep Impurity]
125. Miconazole [orange Book]
126. Schembl13934598
127. Bdbm31772
128. Kbio1_000156
129. Kbio2_001445
130. Kbio2_004013
131. Kbio2_006581
132. Kbio3_001533
133. Miconazole [ep Monograph]
134. Miconazole [usp Impurity]
135. Ninds_000156
136. Hms1568m15
137. Hms2090b21
138. Hms2095m15
139. Hms2232b14
140. Hms3374j10
141. Hms3656e14
142. Hms3712m15
143. Miconazole [usp Monograph]
144. Hy-b0454
145. Tox21_110601
146. Dl-448
147. Nsc170986
148. S2536
149. Stk834405
150. 1-(2,4-dichlorophenyl)-1-[(2,4-dichlorophenyl)methoxy]-2-imidazolylethane
151. Akos001574474
152. Akos016842489
153. Ccg-220067
154. Db01110
155. Ds-1881
156. 1h-imidazole, 1-2-((2,4-dichlorophenyl)-2-((2,4-dichlorophenyl))methoxy)ethyl)-, (+-)-
157. Idi1_000156
158. Ncgc00018294-02
159. Ncgc00018294-04
160. Ncgc00018294-06
161. Ncgc00018294-08
162. 75319-47-0
163. Miconazole 100 Microg/ml In Acetonitrile
164. Nci60_001353
165. Nci60_001380
166. Smr001307249
167. Sbi-0051448.p003
168. Cas-22916-47-8
169. Db-046018
170. Ab00053500
171. Ft-0628942
172. Sw196614-4
173. D00416
174. R18134
175. Ab00053500-23
176. Ab00053500-24
177. Ab00053500-25
178. Ab00053500_26
179. Ab00053500_27
180. Ab00053500_28
181. 216m019
182. A878389
183. Ae-641/01941016
184. Q410534
185. J-014898
186. Sr-01000000271-5
187. Brd-a82396632-001-03-0
188. Brd-a82396632-008-02-7
189. 1-[2,4dichloro-beta-(2,4-dichlorobenzyloxy)phenethyl]imidazole
190. 1-[2,4-dichloro-beta-([2,4-dichlorobenzyl]oxy)-phenethyl]imidazole
191. 1-[2,4-dichloro-beta-(2,4-dichlorobenzyloxy)-phenethyl]imidazole
192. 1-[2,4-dichloro-beta-(2,4-dichlorobenzyloxy)phenethyl]-imidazole
193. 1-[2,4-dichloro-beta-(2,4-dichlorobenzyloxy)phenethyl]imidazole
194. Imidazole,4-dichloro-.beta.-[(2,4-dichlorobenzyl)oxy]phenethyl]-
195. 1-(2-(2,4-dichlorophenyl)-2-((2,4-dichlorophenyl)methoxy)ethyl)-imidazol
196. 1-[2,4-dichloro-.beta.-[(2,4-dichlorobenzyl)oxy]phenethyl]imidazole
197. 1-[2,4-dichloro-beta-([2,4-dichlorobenzyl]oxy)-phenethyl] Imidazole
198. 1-[2-(2,4-dichlorobenzyloxy)-2-(2,4-dichlorophenyl)ethyl]imidazole
199. 1h-imidazole,4-dichlorophenyl)-2-[(2,4-dichlorophenyl)methoxy]ethyl]-
200. Imidazole, 1-(2,4-dichloro-.beta.-((2,4-dichlorobenzyl)oxy)phenethyl)-
201. (+/-)-1-(2,4-dichloro-b-((2,4-dichlorobenzyl)oxy)phenethyl)imidazole
202. 1-[2-(2,4-dichloro-benzyloxy)-2-(2,4-dichloro-phenyl)-ethyl]-1h-imidazole
203. 1-[2-(2,4-dichlorobenzyl)oxy-2-(2,4-dichlorophenyl)ethyl]imidazole;nitric Acid
204. 1-[2-(2,4-dichlorophenyl)-2-[(2,4-dichlorophenyl)-methoxy]ethyl]-1h-imidazole
205. 1-[2-[(2,4-dichlorobenzyl)oxy]-2-(2,4-dichlorophenyl)ethyl]-1h-imidazole #
206. Imidazole, 1-(2-(2,4-dichlorophenyl)-2-((2,4-dichlorophenyl)methoxy)ethyl)- (9ci)
207. 1h-imidazole, 1-2-((2,4-dichlorophenyl)-2-((2,4-dichlorophenyl))methoxy)ethyl)-, (+/-)-
208. Monistat, Daktarin Iv, Miconazolo, Miconazolum, Dactarin, Miconazol, Minostate, Brentan, Florid(nitrate)
| Molecular Weight | 416.1 g/mol |
|---|---|
| Molecular Formula | C18H14Cl4N2O |
| XLogP3 | 5.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 6 |
| Exact Mass | 415.983074 g/mol |
| Monoisotopic Mass | 413.986024 g/mol |
| Topological Polar Surface Area | 27 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 417 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 22 | |
|---|---|
| Drug Name | Miconazole 3 |
| PubMed Health | Miconazole/Zinc Oxide/White Petrolatum (On the skin) |
| Drug Classes | Antifungal |
| Active Ingredient | Miconazole nitrate |
| Dosage Form | Cream |
| Route | Vaginal |
| Strength | 4% |
| Market Status | Over the Counter |
| Company | Taro |
| 2 of 22 | |
|---|---|
| Drug Name | Miconazole 3 combination pack |
| Active Ingredient | Miconazole nitrate |
| Dosage Form | Cream |
| Route | Topical, vaginal |
| Strength | 2%,4% |
| Market Status | Over the Counter |
| Company | Perrigo |
| 3 of 22 | |
|---|---|
| Drug Name | Miconazole 7 |
| Active Ingredient | Miconazole nitrate |
| Dosage Form | Cream |
| Route | Vaginal |
| Strength | 2% |
| Market Status | Over the Counter |
| Company | Actavis Mid Atlantic |
| 4 of 22 | |
|---|---|
| Drug Name | Miconazole 7 combination pack |
| Active Ingredient | Miconazole nitrate |
| Dosage Form | Cream, suppository |
| Route | Topical, vaginal |
| Strength | 2%,100mg |
| Market Status | Over the Counter |
| Company | G And W Labs |
| 5 of 22 | |
|---|---|
| Drug Name | Monistat 1 combination pack |
| Drug Label | MONISTAT 3 Vaginal Suppositories are white to off-white suppositories, each containing the antifungal agent, miconazole nitrate, 1-[2,4-Dichloro--[(2,4-dichlorobenzyl)oxy]phenethyl]-imidazole mononitrate, 200 mg, in a hydrogenated vegetable oil bas... |
| Active Ingredient | Miconazole nitrate |
| Dosage Form | Cream, suppository |
| Route | Topical, vaginal |
| Strength | 2%,1.2gm |
| Market Status | Over the Counter |
| Company | Insight Pharms |
| 6 of 22 | |
|---|---|
| Drug Name | Monistat 3 |
| Active Ingredient | Miconazole nitrate |
| Dosage Form | Cream; Suppository |
| Route | Vaginal |
| Strength | 200mg; 4% |
| Market Status | Over the Counter; Prescription |
| Company | Insight Pharms |
| 7 of 22 | |
|---|---|
| Drug Name | Monistat 3 combination pack |
| Active Ingredient | Miconazole nitrate |
| Dosage Form | Cream |
| Route | Topical, vaginal |
| Strength | 2%,4% |
| Market Status | Over the Counter |
| Company | Insight Pharms |
| 8 of 22 | |
|---|---|
| Drug Name | Monistat 7 |
| Drug Label | ORAVIG (miconazole) buccal tablets are applied topically to the gum once daily and release miconazole as the buccal tablet gradually dissolves [ see Clinical Pharmacology (12.3)].Miconazole is an imidazole antifungal agent and is described chemically... |
| Active Ingredient | Miconazole nitrate |
| Dosage Form | Cream; Suppository |
| Route | Vaginal |
| Strength | 100mg; 2% |
| Market Status | Over the Counter |
| Company | Insight Pharms |
| 9 of 22 | |
|---|---|
| Drug Name | M-zole 3 combination pack |
| PubMed Health | Miconazole |
| Drug Classes | Antifungal, Imidazole |
| Active Ingredient | Miconazole nitrate |
| Dosage Form | Cream, suppository |
| Route | Topical, vaginal |
| Strength | 2%,200mg |
| Market Status | Over the Counter |
| Company | Actavis Mid Atlantic |
| 10 of 22 | |
|---|---|
| Drug Name | Oravig |
| Active Ingredient | Miconazole |
| Dosage Form | Tablet |
| Route | Buccal |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Bioalliance Pharma |
| 11 of 22 | |
|---|---|
| Drug Name | Vusion |
| Active Ingredient | petrolatum, white; Miconazole nitrate; zinc oxide |
| Dosage Form | Ointment |
| Route | Topical |
| Strength | 15%; 81.35%; 0.25% |
| Market Status | Prescription |
| Company | Delcor Asset |
| 12 of 22 | |
|---|---|
| Drug Name | Miconazole 3 |
| PubMed Health | Miconazole/Zinc Oxide/White Petrolatum (On the skin) |
| Drug Classes | Antifungal |
| Active Ingredient | Miconazole nitrate |
| Dosage Form | Cream |
| Route | Vaginal |
| Strength | 4% |
| Market Status | Over the Counter |
| Company | Taro |
| 13 of 22 | |
|---|---|
| Drug Name | Miconazole 3 combination pack |
| Active Ingredient | Miconazole nitrate |
| Dosage Form | Cream |
| Route | Topical, vaginal |
| Strength | 2%,4% |
| Market Status | Over the Counter |
| Company | Perrigo |
| 14 of 22 | |
|---|---|
| Drug Name | Miconazole 7 |
| Active Ingredient | Miconazole nitrate |
| Dosage Form | Cream |
| Route | Vaginal |
| Strength | 2% |
| Market Status | Over the Counter |
| Company | Actavis Mid Atlantic |
| 15 of 22 | |
|---|---|
| Drug Name | Miconazole 7 combination pack |
| Active Ingredient | Miconazole nitrate |
| Dosage Form | Cream, suppository |
| Route | Topical, vaginal |
| Strength | 2%,100mg |
| Market Status | Over the Counter |
| Company | G And W Labs |
| 16 of 22 | |
|---|---|
| Drug Name | Monistat 1 combination pack |
| Drug Label | MONISTAT 3 Vaginal Suppositories are white to off-white suppositories, each containing the antifungal agent, miconazole nitrate, 1-[2,4-Dichloro--[(2,4-dichlorobenzyl)oxy]phenethyl]-imidazole mononitrate, 200 mg, in a hydrogenated vegetable oil bas... |
| Active Ingredient | Miconazole nitrate |
| Dosage Form | Cream, suppository |
| Route | Topical, vaginal |
| Strength | 2%,1.2gm |
| Market Status | Over the Counter |
| Company | Insight Pharms |
| 17 of 22 | |
|---|---|
| Drug Name | Monistat 3 |
| Active Ingredient | Miconazole nitrate |
| Dosage Form | Cream; Suppository |
| Route | Vaginal |
| Strength | 200mg; 4% |
| Market Status | Over the Counter; Prescription |
| Company | Insight Pharms |
| 18 of 22 | |
|---|---|
| Drug Name | Monistat 3 combination pack |
| Active Ingredient | Miconazole nitrate |
| Dosage Form | Cream |
| Route | Topical, vaginal |
| Strength | 2%,4% |
| Market Status | Over the Counter |
| Company | Insight Pharms |
| 19 of 22 | |
|---|---|
| Drug Name | Monistat 7 |
| Drug Label | ORAVIG (miconazole) buccal tablets are applied topically to the gum once daily and release miconazole as the buccal tablet gradually dissolves [ see Clinical Pharmacology (12.3)].Miconazole is an imidazole antifungal agent and is described chemically... |
| Active Ingredient | Miconazole nitrate |
| Dosage Form | Cream; Suppository |
| Route | Vaginal |
| Strength | 100mg; 2% |
| Market Status | Over the Counter |
| Company | Insight Pharms |
| 20 of 22 | |
|---|---|
| Drug Name | M-zole 3 combination pack |
| PubMed Health | Miconazole |
| Drug Classes | Antifungal, Imidazole |
| Active Ingredient | Miconazole nitrate |
| Dosage Form | Cream, suppository |
| Route | Topical, vaginal |
| Strength | 2%,200mg |
| Market Status | Over the Counter |
| Company | Actavis Mid Atlantic |
| 21 of 22 | |
|---|---|
| Drug Name | Oravig |
| Active Ingredient | Miconazole |
| Dosage Form | Tablet |
| Route | Buccal |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Bioalliance Pharma |
| 22 of 22 | |
|---|---|
| Drug Name | Vusion |
| Active Ingredient | petrolatum, white; Miconazole nitrate; zinc oxide |
| Dosage Form | Ointment |
| Route | Topical |
| Strength | 15%; 81.35%; 0.25% |
| Market Status | Prescription |
| Company | Delcor Asset |
Miconazole is indicated for the local treatment of oropharyngeal candidiasis in adult patients and for the adjunctive treatment of diaper dermatitis complicated by candidiasis in immunocompetent patients aged four weeks and older. Miconazole is available as both a suppository and cream for the treatment of vaginal yeast infections and the relief of associated vulvar itching and irritation. Lastly, miconazole cream is effective in treating athlete's foot (tinea pedis), jock itch (tinea cruris), ringworm infections (tinea corporis), pityriasis (formerly tinea) versicolor, and cutaneous candidiasis.
FDA Label
Miconazole is an azole antifungal that functions primarily through inhibition of a specific demethylase within the CYP450 complex. As miconazole is typically applied topically and is minimally absorbed into the systemic circulation following application, the majority of patient reactions are limited to hypersensitivity and cases of anaphylaxis. Patients using intravaginal miconazole products are advised not to rely on contraceptives to prevent pregnancy and sexually transmitted infections, as well as not to use tampons concurrently.
Cytochrome P-450 CYP3A Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP3A. (See all compounds classified as Cytochrome P-450 CYP3A Inhibitors.)
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
14-alpha Demethylase Inhibitors
Compounds that specifically inhibit STEROL 14-DEMETHYLASE. A variety of azole-derived ANTIFUNGAL AGENTS act through this mechanism. (See all compounds classified as 14-alpha Demethylase Inhibitors.)
Cytochrome P-450 CYP2C9 Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP2C9. (See all compounds classified as Cytochrome P-450 CYP2C9 Inhibitors.)
D01AC02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A01 - Stomatological preparations
A01A - Stomatological preparations
A01AB - Antiinfectives and antiseptics for local oral treatment
A01AB09 - Miconazole
A - Alimentary tract and metabolism
A07 - Antidiarrheals, intestinal antiinflammatory/antiinfective agents
A07A - Intestinal antiinfectives
A07AC - Imidazole derivatives
A07AC01 - Miconazole
D - Dermatologicals
D01 - Antifungals for dermatological use
D01A - Antifungals for topical use
D01AC - Imidazole and triazole derivatives
D01AC02 - Miconazole
G - Genito urinary system and sex hormones
G01 - Gynecological antiinfectives and antiseptics
G01A - Antiinfectives and antiseptics, excl. combinations with corticosteroids
G01AF - Imidazole derivatives
G01AF04 - Miconazole
J - Antiinfectives for systemic use
J02 - Antimycotics for systemic use
J02A - Antimycotics for systemic use
J02AB - Imidazole derivatives
J02AB01 - Miconazole
S - Sensory organs
S02 - Otologicals
S02A - Antiinfectives
S02AA - Antiinfectives
S02AA13 - Miconazole
Absorption
Miconazole given to healthy volunteers as a single 50 mg oral tablet produced a mean Cmax of 15.1 16.2 mcg/mL, a mean AUC0-24 of 55.2 35.1 mcg\*h/mL, and a median Tmax of 7 hours (range 2.0-24.1). In these patients measurable plasma concentrations ranged from 0.5 to 0.83 mcg/mL. Topical miconazole is absorbed poorly into the systemic circulation. In pediatric patients aged 1-21 months given multiple topical applications of miconazole ointment for seven days, the plasma miconazole concentration was less than 0.5 ng/mL in 88% of the patients, with the remaining patients having a concentration of 0.57 and 0.58 ng/mL, respectively. Similarly, patients. administered with a vaginal 1200 mg ovule had a mean Cmax of 10.71 ng/mL, mean Tmax of 18.4 hours, and mean AUC0-96 of 477.3 ng\*h/mL.
Route of Elimination
Miconazole is excreted through both urine and feces; less than 1% of unchanged miconazole is recovered in urine.
Volume of Distribution
A 1200 mg miconazole vaginal suppository resulted in a calculated apparent volume of distribution of 95 546 L while a 100 mg vaginal cream yielded an apparent volume of distribution of 10 911L.
Miconazole is metabolized in the liver and does not give rise to any active metabolites.
Miconazole has a terminal half-life of 24 hours.
Miconazole is an azole antifungal used to treat a variety of conditions, including those caused by _Candida_ overgrowth. Unique among the azoles, miconazole is thought to act through three main mechanisms. The primary mechanism of action is through inhibition of the CYP450 14-lanosterol demethylase enzyme, which results in altered ergosterol production and impaired cell membrane composition and permeability, which in turn leads to cation, phosphate, and low molecular weight protein leakage. In addition, miconazole inhibits fungal peroxidase and catalase while not affecting NADH oxidase activity, leading to increased production of reactive oxygen species (ROS). Increased intracellular ROS leads to downstream pleiotropic effects and eventual apoptosis. Lastly, likely as a result of lanosterol demethylation inhibition, miconazole causes a rise in intracellular levels of farnesol. This molecule participates in quorum sensing in _Candida_, preventing the transition from yeast to mycelial forms and thereby the formation of biofilms, which are more resistant to antibiotics. In addition, farnesol is an inhibitor of drug efflux ABC transporters, namely _Candida_ CaCdr1p and CaCdr2p, which may additionally contribute to increased effectiveness of azole drugs.