


1. 4'-n-benzoyl Staurosporine
2. 4'-n-benzoylstaurosporine
3. Benzamide, N-(2,3,9,10,11,12-hexahydro-9-methoxy-8-methyl-1-oxo-8,12-epoxy-1h,8h-2,7b,12a-triazadibenzo(a,g)cyclonona(cde)trinden-10-yl)-n-methyl-, (8alpha,9beta,10beta,12alpha)-
4. Benzoylstaurosporine
5. Cgp 41 251
6. Cgp 41251
7. Cgp-41251
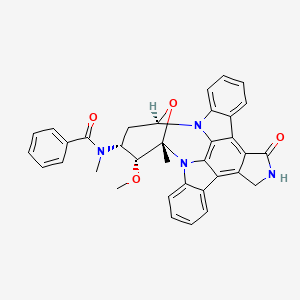
8. N-((9s,10r,11r,13r)-10-methoxy-9-methyl-1-oxo-2,3,10,11,12,13-hexahydro-9,13-epoxy-1h,9h-diindolo(1,2,3-gh:3',2',1'-lm)pyrrolo(3,4-j)(1,7)benzodiazonin-11-yl)-n-methylbenzamide
9. Pkc 412
10. Pkc-412
11. Pkc412
12. Rydapt
1. 120685-11-2
2. Pkc-412
3. Pkc412
4. Cgp 41251
5. 4'-n-benzoylstaurosporine
6. Pkc 412
7. Rydapt
8. Cgp-41251
9. Benzoylstaurosporine
10. N-benzoylstaurosporine
11. Id912s5von
12. Nvp-pkc412
13. Chembl608533
14. Chebi:63452
15. Nsc-656576
16. N-((9s,10r,11r,13r)-10-methoxy-9-methyl-1-oxo-2,3,10,11,12,13-hexahydro-9,13-epoxy-1h,9h-diindolo(1,2,3-gh:3',2',1'-lm)pyrrolo(3,4-j)(1,7)benzodiazonin-11-yl)-n-methylbenzamide
17. N-[(5s,6r,7r,9r)-6-methoxy-5-methyl-14-oxo-6,7,8,9,15,16-hexahydro-5h,14h-5,9-epoxy-4b,9a,15-triazadibenzo[b,h]cyclonona[1,2,3,4-jkl]cyclopenta[e]-as-indacen-7-yl]-n-methylbenzamide
18. Midostaurin [inn]
19. Cgp 41 251
20. Midostaurin [usan:inn]
21. Unii-id912s5von
22. Cgp41251
23. Cgp 41231
24. Rydapt (tn)
25. Midostaurin(pkc412)
26. 4-n-benzoylstaurosporine
27. Midostaurin [mi]
28. Staurosporine, N-benzoyl
29. Midostaurin [jan]
30. Midostaurin [usan]
31. Midostaurin [mart.]
32. Midostaurin [who-dd]
33. Midostaurin (jan/usan/inn)
34. Gtpl5702
35. Schembl8295379
36. Midostaurin [orange Book]
37. Hms3229k17
38. Ex-a1741
39. Bdbm50326053
40. Cgp-41521
41. Mfcd00871372
42. Nsc800791
43. S8064
44. Akos024457372
45. Zinc100013130
46. Ccg-101288
47. Cs-3331
48. Db06595
49. Nsc 656576
50. Nsc-800791
51. Ncgc00241102-01
52. Ncgc00241102-02
53. Ncgc00241102-05
54. Ncgc00484987-03
55. Ac-31929
56. Benzamide, N-(2,3,9,10,11,12-hexahydro-9-methoxy-8-methyl-1-oxo-8,12-epoxy-1h,8h-2,7b,12a-triazadibenzo(a,g)cyclonona(cde)trinden-10-yl)-n-methyl-, (8alpha,9beta,10beta,12alpha)-
57. Hy-10230
58. C71714
59. D05029
60. J-004379
61. Q6842945
62. Brd-k13646352-001-01-2
63. [9s-(9?,10?,11?,13?)]-n-(2,3,10,11,12,13-hexahydro-10-methoxy-9-methyl-1-oxo-9,13-epoxy-1h,9h-diindolo[1,2,3-gh:3',2',1'-lm]pyrrolo[3,4-j][1,7]benzodiazonin-11-yl)-n-methylbenzamide
64. Benzamide, N-((9s,10r,11r,13r)-2,3,9,10,11,12-hexahydro-10-methoxy-9-methyl-1-oxo-9,13-epoxy-1h,9h-diindolo(1,2,3-gh:3',2',1'-lm)pyrrolo(3,4-j)(1,7)benzodiazonin-11-yl)-n-methyl-
65. N-[(2s,3r,4r,6r)-3-methoxy-2-methyl-16-oxo-29-oxa-1,7,17-triazaoctacyclo[12.12.2.1^{2,6}.0^{7,28}.0^{8,13}.0^{15,19}.0^{20,27}.0^{21,26}]nonacosa-8,10,12,14(28),15(19),20(27),21,23,25-nonaen-4-yl]-n-methylbenzamide
66. N-[(2s,3r,4r,6r)-3-methoxy-2-methyl-16-oxo-29-oxa-1,7,17-triazaoctacyclo[12.12.2.12,6.07,28.08,13.015,19.020,27.021,26]nonacosa-8(13),9,11,14,19,21(26),22,24,27-nonaen-4-yl]-n-methyl-benzamide
67. N-[(2s,3r,4r,6r)-3-methoxy-2-methyl-16-oxo-29-oxa-1,7,17-triazaoctacyclo[12.12.2.12,6.07,28.08,13.015,19.020,27.021,26]nonacosa-8,10,12,14,19,21,23,25,27-nonaen-4-yl]-n-methylbenzamide
| Molecular Weight | 570.6 g/mol |
|---|---|
| Molecular Formula | C35H30N4O4 |
| XLogP3 | 4.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 570.22670545 g/mol |
| Monoisotopic Mass | 570.22670545 g/mol |
| Topological Polar Surface Area | 77.7 Ų |
| Heavy Atom Count | 43 |
| Formal Charge | 0 |
| Complexity | 1140 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 1 | |
|---|---|
| Drug Name | RYDAPT |
| Active Ingredient | MIDOSTAURIN |
| Company | NOVARTIS PHARMS CORP (Application Number: N207997. Patents: 7973031, 8222244, 8575146) |
Investigated for use/treatment in adult patients with high-risk acute myeloid leukemia (AML) who are FLT3 mutation-positive, agressive systemic mastocytosis (ASM), systemic mastocytosis with associated hematological neoplasm (SM-AHN), or mast cell leukemia (MCL).
FDA Label
Rydapt is indicated:
- in combination with standard daunorubicin and cytarabine induction and high dose cytarabine consolidation chemotherapy, and for patients in complete response followed by Rydapt single agent maintenance therapy, for adult patients with newly diagnosed acute myeloid leukaemia (AML) who are FLT3 mutation positive (see section 4. 2);
- as monotherapy for the treatment of adult patients with aggressive systemic mastocytosis (ASM), systemic mastocytosis with associated haematological neoplasm (SM AHN), or mast cell leukaemia (MCL).
It targets multiple WT and mutated kinases that, when activated, constitutively stimulate aberrant signalling cascades that lead to malignancies such as AML and ASM. Alternative pharmacodynamic effect of midostaurin in prolonging QTc intervals was not clinically significant in patients with advanced SM or AML when compared to placebo. Midostaurin is therapeutically beneficial as a combination therapy for patients undergoing chemotherapy.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)
L01XE
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EX - Other protein kinase inhibitors
L01EX10 - Midostaurin
Absorption
The time to reach maximum concentration ranges from 1-3 hrs in fasting patients. The maximum concentration and the time it takes to reach this concentration is reduced up to 20% in presence of a standard meal.
Route of Elimination
Accounting for 95% of recovered dose eliminated through fecal excretion, 91% was determined as metabolites and 4% as unchanged parent drug. Remaining 5% of the recovered dose is eliminated via renal excretion.
Volume of Distribution
The Vd of midostaurin is 95.2L. The parent drug and its main metabolites (CGP62221, CGP52421) are distributed in plasma in vitro.
Clearance
The clearance values of during the initial formation of metabolites are 1.47 L/h for CGP62221 metabolite and 0.501 L/h for CGP52421. 28 days following the oral administration of midostaurin, the clearance of CGP52421 may increase up to 5.2 fold in a recommended dose of 25 mg, resulting in a 2.1- to 2.5-fold increase in total clearance of midostaurin.
Midostaurin is primarily metabolized into CGP62221 and CGP52421 via hepatic CYP3A4 enzymatic activity. The metabolism of CGP62221 takes place initially in a linear relationship whereas CGP52421 formation is an inducible process.
Elimination half life is approximately 21 hrs for midostaurin, 32 hrs for CGP62221 and 482 hrs for CGP52421.
It potently inhibits multiple receptor tyrosine kinases. Midostaurin and its major active metabolites CGP62221 and CGP52421 inhibit the activity of protein kinase C alpha (PKCalpha), VEGFR2, KIT, PDGFR and WT and/or mutant FLT3 tyrosine kinases. Inhibition of FLT3 receptor signalling cascades induces apoptosis of target leukemia cells expressing target receptors and mast cells, in addition to its antiproliferative activity toward multiple cancer cell lines. Midostaurin also interacts with organic anion transporter (OATP) 1A1 and multidrug resistance protein (MRP)-2 according to preliminary in vitro studies.