
1. Mifgyne
2. Mifegyne
3. Mifeprex
4. R 38486
5. R-38486
6. R38486
7. Ru 38486
8. Ru 486
9. Ru-38486
10. Ru-486
11. Ru38486
12. Ru486
13. Zk 98296
14. Zk-98296
15. Zk98296
1. 84371-65-3
2. Mifegyne
3. Mifeprex
4. Ru-486
5. Korlym
6. Ru486
7. Ru 486
8. Mifepriston
9. Corlux
10. Ru 38486
11. Ru-38486
12. Mls000069785
13. 320t6rnw1f
14. Vgx-410c
15. Chebi:50692
16. Vgx-410
17. Nsc-759862
18. Ncgc00025179-05
19. Smr000058481
20. Mifepristonum [latin]
21. Mifepristona [spanish]
22. Dsstox_cid_3322
23. C-1073
24. Dsstox_rid_76976
25. Dsstox_gsid_23322
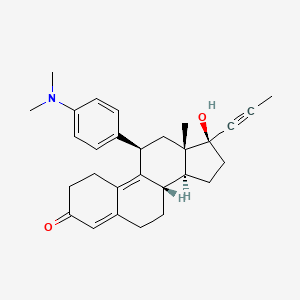
26. (11beta,17beta)-11-[4-(dimethylamino)phenyl]-17-hydroxy-17-(1-propyn-1-yl)estra-4,9-dien-3-one
27. (8s,11r,13s,14s,17s)-11-(4-(dimethylamino)phenyl)-17-hydroxy-13-methyl-17-(prop-1-yn-1-yl)-1,2,6,7,8,11,12,13,14,15,16,17-dodecahydro-3h-cyclopenta[a]phenanthren-3-one
28. (8s,11r,13s,14s,17s)-11-(4-dimethylaminophenyl)-17-hydroxy-13-methyl-17-prop-1-ynyl-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-3-one
29. (8s,11r,13s,14s,17s)-11-[4-(dimethylamino)phenyl]-17-hydroxy-13-methyl-17-prop-1-ynyl-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-3-one
30. 11-(4-dimethylamino-phenyl)-17-hydroxy-13-methyl-17-prop-1-ynyl-1,2,6,7,8,11,12,13,14,15,16,17-dodec Ahydro-cyclopenta[a]phenanthren-3-one
31. Mifepristona
32. Mifepristonum
33. (11beta,17beta)-11-[4-(dimethylamino)phenyl]-17-hydroxy-17-prop-1-yn-1-ylestra-4,9-dien-3-one
34. Ru486 (tetramethyl-rhodamine Conjugated)
35. Mifepristone [usan:inn:ban]
36. Hsdb 6841
37. Sr-01000076011
38. R 38486
39. Brn 5779404
40. Unii-320t6rnw1f
41. Pictovir
42. Ru 486-6
43. Ccris 9332
44. 1nhz
45. 11beta-(p-(dimethylamino)phenyl)-17beta-hydroxy-17-(1-propynyl)estra-4,9-dien-3-one
46. Mifeprex (tn)
47. Pictovir (tm)
48. Prestwick_570
49. Cas-84371-65-3
50. Korlym (tn)
51. 2w8y
52. Mifepristone (mifeprex)
53. Opera_id_562
54. Mifepristone, >=98%
55. Prestwick0_000299
56. Prestwick1_000299
57. Prestwick2_000299
58. Prestwick3_000299
59. Spectrum5_002045
60. Mifepristone [mi]
61. Mifepristone [inn]
62. Mifepristone [jan]
63. Mifepristone [hsdb]
64. Mifepristone [usan]
65. 11beta-(4-(dimethylamino)phenyl)-17beta-hydroxy-17-(1-propynyl)estra-4,9-dien-3-on
66. 17-beta-hydroxy-11-beta-(4-dimethylaminophenyl-1)-17-alpha-(prop-1-ynyl)oestra-4,9-dien-3-one
67. Mifepristone [vandf]
68. Bidd:pxr0123
69. Lopac0_000801
70. Schembl16087
71. Bspbio_000238
72. Mifepristone [mart.]
73. 11beta-(4-(dimethylamino)phenyl)-17-hydroxy-21-methyl-19-nor-17alpha-pregna-4,9-dien-20-m-3-on
74. 11beta-(4-(n,n-dimethylamino)phenyl)-17alpha-(prop-1-ynyl)-delta4,9-estradiene-17beta-ol-3-one
75. Mls001074115
76. Mls001424271
77. (non-labelled)mifepristone-d3
78. Mifepristone [usp-rs]
79. Mifepristone [who-dd]
80. Spbio_002457
81. Ru-486; Mifepristone
82. Bpbio1_000262
83. Chembl438575
84. Gtpl2805
85. Mifepristone (jan/usan/inn)
86. Chembl1276308
87. Dtxsid5023322
88. Bdbm18627
89. Hms1568l20
90. Hms2052l05
91. Hms2090l22
92. Hms2095l20
93. Hms2230p21
94. Hms3262b03
95. Hms3412d17
96. Hms3649j08
97. Hms3676d17
98. Hms3712l20
99. Hms3884d12
100. Mifepristone [orange Book]
101. 11.beta.-(p-(dimethylamino)phenyl)-17.beta.-hydroxy-17-(1-propynyl)estra-4,9-dien-3-one
102. Act02598
103. Bcp02145
104. Zinc3831128
105. Tox21_110952
106. Tox21_301841
107. Tox21_500801
108. Bdbm50072024
109. Hsci1_000369
110. S2606
111. Vx-410
112. Akos015895416
113. Tox21_110952_1
114. Ccg-101164
115. Ci-1073
116. Cs-1435
117. Db00834
118. Lp00801
119. Nc00414
120. Nsc 759862
121. Sdccgsbi-0050778.p002
122. Mifepristone 1.0 Mg/ml In Acetonitrile
123. Ncgc00025179-06
124. Ncgc00025179-07
125. Ncgc00025179-08
126. Ncgc00025179-09
127. Ncgc00025179-12
128. Ncgc00025179-13
129. Ncgc00025179-23
130. Ncgc00179632-01
131. Ncgc00255152-01
132. Ncgc00261486-01
133. (11beta,17beta)-11-(4-(dimethylamino)phenyl)-17-hydroxy-17-(1-propynyl)-estra-4,9-dien-3-one
134. As-13938
135. Cpd000058481
136. Estra-4,9-dien-3-one, 11-(4-(dimethylamino)phenyl)-17-hydroxy-17-(1-propynyl)-, (11-beta,17-beta)-
137. Hy-13683
138. Ru486;c-1073
139. Eu-0100801
140. C07652
141. D00585
142. M 8046
143. 371m653
144. A840767
145. Q411240
146. Sr-01000721888
147. Q-201405
148. Sr-01000076011-1
149. Sr-01000076011-3
150. Sr-01000076011-5
151. Sr-01000076011-9
152. Sr-01000721888-4
153. Brd-k37270826-001-04-5
154. Brd-k37270826-001-31-8
155. Mifepristone, United States Pharmacopeia (usp) Reference Standard
156. (11?,17?)-11-[4-(dimethylamino)phenyl]-17-hydroxy-17-(1-propynyl)-estra-4,9-dien-3-one
157. 11.beta.-[4-(dimethylamino)phenyl]-17.beta.-hydroxy-17-(1-propynyl)estra-4,9-dien-3-one
158. 11beta-(4-dimethylamino)phenyl-17beta-hydroxy-17-(1-propynyl)estra-4,9-dien-3-one
159. 11ss-[p-(dimethylamino)fenyl]-17ss-hydroxy- 17-(1-propynyl)estra-4,9-dien-3-on
160. (10s,11s,14s,15s,17r)-17-[4-(dimethylamino)phenyl]-14-hydroxy-15-methyl-14-(prop-1-yn-1-yl)tetracyclo[8.7.0.0;{2,7}.0;{11,15}]heptadeca-1,6-dien-5-one
161. (10s,11s,14s,15s,17r)-17-[4-(dimethylamino)phenyl]-14-hydroxy-15-methyl-14-(prop-1-yn-1-yl)tetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-1,6-dien-5-one
162. (11.beta.,17.beta.)-11-(4-(dimethylamino)phenyl)-17-hydroxy-17-(1-propynyl)-estra-4,9-dien-3-one
163. (11r,13s,14s,17s)-11-(4-dimethylamino-phenyl)-17-hydroxy-13-methyl-17-prop-1-ynyl-1,2,6,7,8,11,12,13,14,15,16,17-dodecahydro-cyclopenta[a]phenanthren-3-one
164. (8s,11r,13s,14s,17s)-11-[4-(dimethylamino)phenyl]-13-methyl-17-oxidanyl-17-prop-1-ynyl-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-3-one
165. 122742-25-0
166. 83203-42-3
167. Estra-4,9-dien-3-one, 11-[4-(dimethylamino)phenyl]-17-hydroxy-17-(1-propyn-1-yl)-, (11.beta.,17.beta.)-
168. Estra-4,9-dien-3-one, 11-[4-(dimethylamino)phenyl]-17-hydroxy-17-(1-propynyl)-, (11b,17b)-
| Molecular Weight | 429.6 g/mol |
|---|---|
| Molecular Formula | C29H35NO2 |
| XLogP3 | 3.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 429.266779359 g/mol |
| Monoisotopic Mass | 429.266779359 g/mol |
| Topological Polar Surface Area | 40.5 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 921 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Korlym |
| PubMed Health | Mifepristone (By mouth) |
| Drug Classes | Antiglucocorticoid, Antiprogesterone |
| Drug Label | Korlym (mifepristone) is a cortisol receptor blocker for oral administration. The chemical name of mifepristone is 11-(4-dimethylaminophenyl)-17-hydroxy-17-(1-propynyl)-estra-4, 9-dien-3-one. The chemical formula is C29H35NO2; the molecular wei... |
| Active Ingredient | Mifepristone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 300mg |
| Market Status | Prescription |
| Company | Corcept Therap |
| 2 of 4 | |
|---|---|
| Drug Name | Mifeprex |
| Active Ingredient | Mifepristone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg |
| Market Status | Prescription |
| Company | Danco Labs |
| 3 of 4 | |
|---|---|
| Drug Name | Korlym |
| PubMed Health | Mifepristone (By mouth) |
| Drug Classes | Antiglucocorticoid, Antiprogesterone |
| Drug Label | Korlym (mifepristone) is a cortisol receptor blocker for oral administration. The chemical name of mifepristone is 11-(4-dimethylaminophenyl)-17-hydroxy-17-(1-propynyl)-estra-4, 9-dien-3-one. The chemical formula is C29H35NO2; the molecular wei... |
| Active Ingredient | Mifepristone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 300mg |
| Market Status | Prescription |
| Company | Corcept Therap |
| 4 of 4 | |
|---|---|
| Drug Name | Mifeprex |
| Active Ingredient | Mifepristone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg |
| Market Status | Prescription |
| Company | Danco Labs |
Abortifacient Agents, Steroidal; Contraceptives, Oral, Synthetic; Contraceptives, Postcoital, Synthetic; Hormone Antagonists; Luteolytic Agents; Menstruation-Inducing Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Mifepristone is indicated in combination with misoprostol for the medical termination of intrauterine pregnancy of 49 days duration or less. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1971
Confirmed or suspected ectopic pregnancy, undiagnosed adnexal mass, or IUD currently in place. Chronic adrenal failure or concurrent long-term corticosteroid therapy. Known hypersensitivity to mifepristone, misoprostol, or other prostaglandins. Hemorrhagic disorders, inherited porphyrias, or concurrent anticoagulant therapy.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3148
Vaginal bleeding that is heavier than associated with a normal menses occurs in almost all women receiving mifepristone and misoprostol. Based on clinical studies, bleeding or spotting should be expected for an average of 9-16 days. ... Excessive bleeding may require treatment with vasoconstrictors, saline infusions, and/or blood transfusions or curettage.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3148
Severe vaginal bleeding may occur following spontaneous, surgical, or medical abortion (including following mifepristone administration). Prolonged heavy vaginal bleeding (i.e. soaking through 2 thick full-size sanitary pads per hour for 2 consecutive hours) may be a sign of incomplete abortion or other complications, and prompt medical or surgical intervention maybe required to prevent the development of hypovolemic shock. Patients should be advised to seek immediate medical attention if prolonged heavy vaginal bleeding or syncope occurs following mifepristone administration.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3148
Serious bacterial infections (including very rare cases of fatal septic shock) have been reported following mifepristone administration; a causal relationship to the mifepristone-misoprostol regimen has not been established. Clinicians should consider the possibility of infection if sustained fever (temperature of 38 degrees C or higher persisting for more than 4 hours), severe abdominal pain, or pelvic tenderness occurs within several days of medical abortion. Atypical presentations of serious infection and sepsis (i.e., presence of significant leukocytosis, tachycardia, or hemoconcentration without fever, severe abdominal pain, pelvic tenderness) may also occur.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3148
For more Drug Warnings (Complete) data for MIFEPRISTONE (14 total), please visit the HSDB record page.
For the medical termination of intrauterine pregnancy through 49 days' pregnancy. Also indicated to control hyperglycemia secondary to hypercortisolism in adult patients with endogenous Cushing's syndrome who have type 2 diabetes mellitus or glucose intolerance and are not candidates for surgery or have had unsuccessful surgery.
FDA Label
Treatment of endometriosis
Treatment of hypercortisolism (Cushing's syndrome) of endogenous origin
Treatment of leiomyoma of uterus
Mifepristone is a synthetic steroid with antiprogestational effects indicated for the medical termination of intrauterine pregnancy through 49 days' pregnancy. Doses of 1 mg/kg or greater of mifepristone have been shown to antagonize the endometrial and myometrial effects of progesterone in women. During pregnancy, the compound sensitizes the myometrium to the contraction-inducing activity of prostaglandins. Mifepristone also exhibits antiglucocorticoid and weak antiandrogenic activity. The activity of the glucocorticoid dexamethasone in rats was inhibited following doses of 10 to 25 mg/kg of mifepristone. Doses of 4.5 mg/kg or greater in human beings resulted in a compensatory elevation of adrenocorticotropic hormone (ACTH) and cortisol.
Contraceptives, Oral, Synthetic
Oral contraceptives which owe their effectiveness to synthetic preparations. (See all compounds classified as Contraceptives, Oral, Synthetic.)
Luteolytic Agents
Chemical compounds that cause LUTEOLYSIS or degeneration of the CORPUS LUTEUM. (See all compounds classified as Luteolytic Agents.)
Contraceptives, Postcoital, Synthetic
Postcoital contraceptives which owe their effectiveness to synthetic preparations. (See all compounds classified as Contraceptives, Postcoital, Synthetic.)
Abortifacient Agents, Steroidal
Steroidal compounds with abortifacient activity. (See all compounds classified as Abortifacient Agents, Steroidal.)
Menstruation-Inducing Agents
Chemical compounds that induce menstruation either through direct action on the reproductive organs or through indirect action by relieving another condition of which amenorrhea is a secondary result. (From Dorland, 27th ed) (See all compounds classified as Menstruation-Inducing Agents.)
Hormone Antagonists
Chemical substances which inhibit the function of the endocrine glands, the biosynthesis of their secreted hormones, or the action of hormones upon their specific sites. (See all compounds classified as Hormone Antagonists.)
G03XB01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
G - Genito urinary system and sex hormones
G03 - Sex hormones and modulators of the genital system
G03X - Other sex hormones and modulators of the genital system
G03XB - Progesterone receptor modulators
G03XB01 - Mifepristone
Absorption
The absolute bioavailability of a 20 mg oral dose is 69%
Route of Elimination
Fecal: 83%; Renal: 9%.
The absolute bioavailability of oral mifepristone is 69%.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1971
Protein binding: Very high (98%); predominantly to albumin and alpha1- acid glycoprotein.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1971
Time to peak concentration: 90 minutes after a 600 mg oral dose.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1971
Peak plasma concentration: 1.98 mg/L following a single 600 mg oral dose.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1971
Fecal; 83% of a 600 mg dose over 11 days. Renal; 9% of a 600 mg dose over 11 days.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1971
Hepatic. Hepatic, by Cytochrome P450 3A4 isoenzyme to the N-monodemethylated metabolite (RU 42 633); RU 42 698, which results from the loss of two methyl groups from position 11 beta; and RU 42 698, which results from terminal hydroxylation of the 17–propynyl chain.
Hepatic, by Cytochrome P450 3A4 isoenzyme to the N-monodemethylated metabolite (RU 42 633); RU 42 698, which results from terminal hydroxylation of the 17-propynyl chain.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1971
Mifepristone has known human metabolites that include 17alpha-hydroxymifepristone and Monodemethylated mifepristone.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
18 hours
Terminal: 18 hours; begins slowly and becomes more rapid with time.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1971
The anti-progestational activity of mifepristone results from competitive interaction with progesterone at progesterone-receptor sites. Based on studies with various oral doses in several animal species (mouse, rat, rabbit and monkey), the compound inhibits the activity of endogenous or exogenous progesterone. The termination of pregnancy results. In the treatment of Cushing's syndrome, Mifepristone blocks the binding of cortisol to its receptor. It does not decrease cortisol production but reduces the effects of excess cortisol, such as high blood sugar levels.
Mifepristone competitively inhibits the actions of progesterone at progesterone-receptor sites, resulting in termination of pregnancy.The combination of mifepristone and misoprostol causes expulsion of the products of conception through decidual necrosis, myometrial contractions, and cervical softening.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 1971
When administered in the early stages of pregnancy, mifepristone causes decidual breakdown by blockade of uterine progesterone receptors. This leads to detachment of the blastocyte, which decreases hCG production. This in turn causes a decrease in progesterone secretion from the corpus luteum, which further accentuates decidual breakdown. Decreased endogenous progesterone coupled with blockade of progesterone receptors in the uterus increases prostaglandin levels and sensitizes the myometrium to the contractile actions of prostaglandins.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1622
In addition, mifepristone promotes uterine contractions and softening of the cervix and sensitizes the myometrium to effects of prostaglandins (e.g., misoprostol) that stimulate uterine contraction and expulsion of the products of conception. In the absence of progesterone, mifepristone acts as a partial progestin agonist. At dosages higher than those used for termination of pregnancy, mifepristone also exhibits antiglucocorticoid activity. The drug also has been shown to have weak antiandrogenic activity.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3148