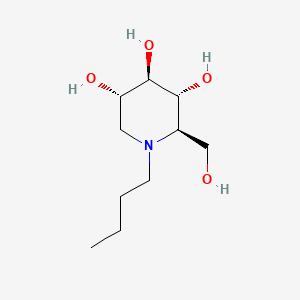

1. Butyldeoxynojirimycin
2. N-(n-butyl)deoxy-nojirimycin
3. N-(n-butyl)deoxynojirimycin
4. N-butyl Deoxynojirimycin
5. N-butyldeoxynojirimycin
6. Ogt 918
7. Ogt-918
8. Sc 48334
9. Sc-48334
10. Zavesca
1. 72599-27-0
2. N-butyldeoxynojirimycin
3. Zavesca
4. Butyldeoxynojirimycin
5. Nb-dnj
6. N-butylmoranoline
7. (2r,3r,4r,5s)-1-butyl-2-(hydroxymethyl)piperidine-3,4,5-triol
8. N-butyl-1-deoxynojirimycin
9. Budnj
10. N-butyl Deoxynojirimycin
11. N-(n-butyl)deoxynojirimycin
12. Ogt 918
13. Sc-48334
14. Ogt-918
15. N-butyl-dnj
16. Sc 48334
17. 3,4,5-piperidinetriol, 1-butyl-2-(hydroxymethyl)-, (2r,3r,4r,5s)-
18. N-butyldeoxynojirimycin.hcl
19. Adn3s497az
20. Chembl1029
21. Chebi:50381
22. 72599-27-0 (free Base)
23. N-butyl Dnj
24. Miglustat [usan]
25. Dsstox_cid_25618
26. Dsstox_rid_81006
27. Dsstox_gsid_45618
28. Miglustatum
29. Vevesca
30. Zavesca (tn)
31. Cas-72599-27-0
32. N-bu-dnj
33. N-butyl-deoxynojirimycin
34. Nbv
35. Unii-adn3s497az
36. Sc48334
37. Brazaves
38. N-(n-butyl)deoxy-nojirimycin
39. Miglustat [usan:inn:ban]
40. D-glucitol, 1,5-(butylimino)-1,5-dideoxy-
41. Ncgc00018140-02
42. Brazaves (tn)
43. Mfcd00272581
44. Miglustat [inn]
45. Miglustat [jan]
46. Miglustat [mi]
47. 1,5-dideoxy-1,5-n-butylimino-d-glucitol
48. Miglustat [vandf]
49. Miglustat [mart.]
50. Miglustat [who-dd]
51. N-(n-butyl)-1,5-dideoxy-1,5-imino-d-glucitol
52. Miglustat [ema Epar]
53. Miglustat (jan/usan/inn)
54. Schembl246893
55. Ogt918n-butyldeoxynojirimycin
56. Gtpl4841
57. Ogt918
58. Miglustat [orange Book]
59. Dtxsid6045618
60. Bdbm18355
61. Hms2090n20
62. Zinc3794711
63. Tox21_110830
64. Akos028109118
65. Tox21_110830_1
66. Db00419
67. 3,4,5-piperidinetriol, 1-butyl-2-(hydroxymethyl)-, (2r-(2alpha,3beta,4alpha,5beta))-
68. Ncgc00024452-03
69. Ncgc00024452-04
70. Hy-17020
71. N-(n-butyl)-1-deoxynojirimycin Min. 99%
72. N-butyldeoxynojirimycin, Film (dried In Situ)
73. D05032
74. P16976
75. Ab00489939-10
76. 1,5-(butylimino)-1,5 Dideoxy,d-glucitol
77. A850985
78. Q425911
79. Sr-01000000043
80. Sr-01000000043-2
81. W-203639
82. 1,5-(butylimino)-1,5-dideoxy-d-glucitol(2r,3r,4r,5s)-1-butyl-2-(hydroxymethyl)-3,4,5-piperidinetriol
83. 134282-77-2
| Molecular Weight | 219.28 g/mol |
|---|---|
| Molecular Formula | C10H21NO4 |
| XLogP3 | -0.6 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Exact Mass | 219.14705815 g/mol |
| Monoisotopic Mass | 219.14705815 g/mol |
| Topological Polar Surface Area | 84.2 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 190 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Zavesca |
| PubMed Health | Miglustat (By mouth) |
| Drug Classes | Endocrine-Metabolic Agent |
| Drug Label | Zavesca (miglustat capsules, 100 mg) is an inhibitor of the enzyme glucosylceramide synthase, which is a glucosyl transferase enzyme responsible for the first step in the synthesis of most glycosphingolipids. Zavesca is an N-alkylated imino sugar, a... |
| Active Ingredient | Miglustat |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 100mg |
| Market Status | Prescription |
| Company | Actelion Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Zavesca |
| PubMed Health | Miglustat (By mouth) |
| Drug Classes | Endocrine-Metabolic Agent |
| Drug Label | Zavesca (miglustat capsules, 100 mg) is an inhibitor of the enzyme glucosylceramide synthase, which is a glucosyl transferase enzyme responsible for the first step in the synthesis of most glycosphingolipids. Zavesca is an N-alkylated imino sugar, a... |
| Active Ingredient | Miglustat |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 100mg |
| Market Status | Prescription |
| Company | Actelion Pharms |
For the treatment of adult patients with mild to moderate type 1 (nonneuropathic) Gaucher's disease for whom enzyme replacement therapy is not a therapeutic option (e.g. due to constraints such as allergy, hypersensitivity, or poor venous access). Now approved in some countries for the treatment of progressive neurological symptoms in adult and pediatric patients with Niemann-Pick disease type C (NP-C).
FDA Label
Miglustat Gen. Orph is indicated for the oral treatment of adult patients with mild to moderate type 1 Gaucher disease.
Miglustat Gen. Orph may be used only in the treatment of patients for whom enzyme replacement therapy is unsuitable.
Miglustat Gen. Orph is indicated for the treatment of progressive neurological manifestations in adult patients and paediatric patients with Niemann-Pick type C disease.
Yargesa is indicated for the oral treatment of adult patients with mild to moderate type 1 Gaucher disease.
Yargesa may be used only in the treatment of patients for whom enzyme replacement therapy is unsuitable.
Yargesa is indicated for the treatment of progressive neurological manifestations in adult patients and paediatric patients with Niemann-Pick type C disease.
Zavesca is indicated for the oral treatment of adult patients with mild to moderate type-1 Gaucher disease. Zavesca may be used only in the treatment of patients for whom enzyme replacement therapy is unsuitable.
Zavesca is indicated for the treatment of progressive neurological manifestations in adult patients and paediatric patients with Niemann-Pick type-C disease.
Miglustat Dipharma is indicated for the oral treatment of adult patients with mild to moderate type 1 Gaucher disease.
Miglustat Dipharma may be used only in the treatment of patients for whom enzyme replacement therapy is unsuitable.
Miglustat Dipharma is indicated for the treatment of progressive neurological manifestations in adult patients and paediatric patients with Niemann-Pick type C disease.
Miglustat, an N-alkylated imino sugar, is a synthetic analogue of D-glucose. Miglustat is an inhibitor of the enzyme glucosylceramide synthase, which is a glucosyl transferase enzyme responsible for catalyzing the formation of glucosylceramide (glucocerebroside). Glucosylceramide is a substrate for the endogenous glucocerebrosidase, an enzyme that is deficient in Gaucher's disease. The accumulation of glucosylceramide due to the absence of glucocerebrosidase results in the storage of this material in the lysosomes of tissue macrophages, leading to widespread pathology due to infiltration of lipid-engorged macrophages in the viscera, lymph nodes, and bone marrow. This results in secondary hematologic consequences including sever anemia and thrombocytopenia, in addition to the characteristic progressive hepatosplenomegaly, as well as skeletal complications including osteonecrosis and osteopenia with secondary pathological fractures.
Anti-HIV Agents
Agents used to treat AIDS and/or stop the spread of the HIV infection. These do not include drugs used to treat symptoms or opportunistic infections associated with AIDS. (See all compounds classified as Anti-HIV Agents.)
Glycoside Hydrolase Inhibitors
Compounds that inhibit or block the activity of GLYCOSIDE HYDROLASES such as ALPHA-AMYLASES and ALPHA-GLUCOSIDASES. (See all compounds classified as Glycoside Hydrolase Inhibitors.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
A16AX06
A16AX06
A16AX06
A16AX06
A16AX06
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A16 - Other alimentary tract and metabolism products
A16A - Other alimentary tract and metabolism products
A16AX - Various alimentary tract and metabolism products
A16AX06 - Miglustat
Absorption
Mean oral bioavailability is 97%.
There is no evidence that miglustat is metabolized in humans.
The effective half-life of miglustat is approximately 6 to 7 hours.
Miglustat functions as a competitive and reversible inhibitor of the enzyme glucosylceramide synthase, the initial enzyme in a series of reactions which results in the synthesis of most glycosphingolipids. The goal of treatment with miglustat is to reduce the rate of glycosphingolipid biosynthesis so that the amount of glycosphingolipid substrate is reduced to a level which allows the residual activity of the deficient glucocerebrosidase enzyme to be more effective (substrate reduction therapy), reducing the accumulation of glucocerebroside in macrophages. In vitro and in vivo studies have shown that miglustat can reduce the synthesis of glucosylceramide-based glycosphingolipids. In clinical trials, miglustat improved liver and spleen volume, as well as hemoglobin concentration and platelet count. Inhibition of glycosphingolipid synthesis has also shown to reduce intracellular lipid storage, improve fluid-phase endosomal uptake and normalize lipid transport in peripheral blood B lymphocytes of NP-C patients, which results in a decrease in the potentially neurotoxic accumulation of gnagliosides GM2 and GM3, lactosylceramide and glucosylceramide, possibly preventing further neuronal damage. Other studies have also suggested that miglustat may indirectly modulate intracellular calcium homeostasis through its effects on glucosylceramide levels, and evidence has shown that an initiating factor in the pathogenesis of NP-C may be impaired calcium homeostasis related to sphingosine storage. Therefore, the effect that miglustat exerts on intracellular calcium levels may influence an important underlying pathogenic mechanism of NP-C.