

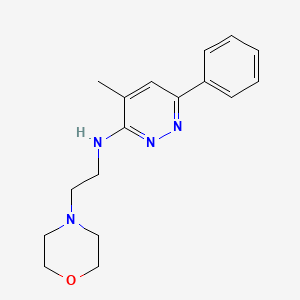

1. 3-(2-morpholino-ethylamino)-4-methyl-6-phenyl Pyridazine, Dihydrochloride
2. 3-(morpholinoethyl)amino-4-methyl-6-phenylpyridazine
3. Agr 1240
4. Cantor
5. Minaprine Dihydrochloride
6. Minaprine Hydrochloride
1. Cantor
2. 25905-77-5
3. Minaprinum
4. Minaprina
5. Minaprinum [inn-latin]
6. Minaprina [inn-spanish]
7. Agr 1240
8. N-(4-methyl-6-phenyl-3-pyridazinyl)-4-morpholineethanamine
9. 4-morpholineethanamine, N-(4-methyl-6-phenyl-3-pyridazinyl)-
10. 4-(2-((4-methyl-6-phenyl-3-pyridazinyl)amino)ethyl)morpholine
11. 4-methyl-3-(2-morpholinoethylamino)-6-phenylpyridazin
12. Agr-1240
13. 4-methyl-n-(2-morpholin-4-ylethyl)-6-phenylpyridazin-3-amine
14. 00u7gx0nlm
15. Cb-30038
16. 4-methyl-n-(2-morpholinoethyl)-6-phenylpyridazin-3-amine
17. Chembl278819
18. Chebi:51038
19. 25905-77-5 (free Base)
20. Brantur
21. 4-methyl-n-[2-(morpholin-4-yl)ethyl]-6-phenylpyridazin-3-amine
22. 4-[2-[(4-methyl-6-phenyl-3-pyridazinyl)amino]ethyl]morpholine
23. Minaprin Dihydrochloride
24. Chembl536932
25. Cantor (tn)
26. Cb 30038
27. Minaprine (usan/inn)
28. Einecs 247-329-8
29. Cas-25953-17-7
30. Unii-00u7gx0nlm
31. Minaprine [usan:inn:ban]
32. Spectrum_001334
33. Minaprine [inn]
34. Minaprine [mi]
35. Minaprine [usan]
36. Prestwick0_000066
37. Prestwick1_000066
38. Prestwick2_000066
39. Prestwick3_000066
40. Spectrum2_001407
41. Spectrum3_001445
42. Spectrum4_000418
43. Spectrum5_001593
44. Minaprine [who-dd]
45. Schembl49371
46. Bspbio_000251
47. Bspbio_002909
48. Kbiogr_000716
49. Kbioss_001814
50. Divk1c_000063
51. Spbio_001454
52. Spbio_002172
53. Bpbio1_000277
54. Dtxsid5048477
55. Kbio1_000063
56. Kbio2_001814
57. Kbio2_004382
58. Kbio2_006950
59. Kbio3_002409
60. Ninds_000063
61. Hy-b0884
62. (r)-benzyl2-oxooxetan-3-ylcarbamate
63. Bdbm50074289
64. Zinc19796082
65. Akos024287006
66. Cs-4346
67. Db00805
68. Idi1_000063
69. Ncgc00016795-01
70. Ncgc00016795-02
71. Ncgc00016795-03
72. Ncgc00016795-04
73. Sbi-0051647.p002
74. Db-046791
75. Ab00053599
76. Ft-0630519
77. D05039
78. Ab00053599_13
79. L001202
80. Q6863173
81. Brd-k02867583-300-05-5
82. Brd-k02867583-300-06-3
83. 3-(2-morpholinoethylamino)-4-methyl-6-phenylpyridazine
84. 4-methyl-n-[2-(4-morpholinyl)ethyl]-6-phenyl-3-pyridazinamine #
85. (4-methyl-6-phenyl-pyridazin-3-yl)-(2-morpholin-4-yl-ethyl)-amine; Hydrochloride
| Molecular Weight | 298.4 g/mol |
|---|---|
| Molecular Formula | C17H22N4O |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 5 |
| Exact Mass | 298.17936134 g/mol |
| Monoisotopic Mass | 298.17936134 g/mol |
| Topological Polar Surface Area | 50.3 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 316 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of depression
Minaprine is an amino-phenylpyridazine antidepressant reported to be relatively free of cardiotoxicity, drowsiness, and weight gain. Similar to other antidepressant treatments, minaprine attenuates the beta-adrenergic receptor function. Studies have also shown that minaprine improves memory consolidation and that repeated drug administration leads to potentiation of this effect. Moreover, the effects of minaprine on memory consolidation are related to its dopaminergic action.
Antidepressive Agents
Mood-stimulating drugs used primarily in the treatment of affective disorders and related conditions. Several MONOAMINE OXIDASE INHIBITORS are useful as antidepressants apparently as a long-term consequence of their modulation of catecholamine levels. The tricyclic compounds useful as antidepressive agents (ANTIDEPRESSIVE AGENTS, TRICYCLIC) also appear to act through brain catecholamine systems. A third group (ANTIDEPRESSIVE AGENTS, SECOND-GENERATION) is a diverse group of drugs including some that act specifically on serotonergic systems. (See all compounds classified as Antidepressive Agents.)
N - Nervous system
N06 - Psychoanaleptics
N06A - Antidepressants
N06AX - Other antidepressants
N06AX07 - Minaprine
Hepatic. Cytochrome P4502D is responsible for the 4-hydroxylation of minaprine to 4-hydroxyminaprine.
Minaprine has known human metabolites that include 4-Hydroxyminaprine.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Minaprine binds to serotonin type 2 receptors and to dopamine D1 and D2 type receptors. It also binds to the serotonin reuptake pump. Therefore, minaprine blocks the reuptake of both dopamine and serotonin. It is also, to a slight degree, cholinomimetic. Thus it may exhibit both mood-brightening and nootropic properties. It also acts as a reversible inhibitor of MAO-A (RIMA).It has also been found to inhibit acetylcholinesterase.