


1. Maidimeisu
2. Midecamin
3. Midecamycin
4. Midecamycin Diacetate
5. Midekamycin
6. Midekamycin Acetate
7. Mosil
8. Mydecamycin
9. Myoxam
10. Neoisomidecamycin
11. Normicina
12. Sf 837
13. Sf-837
1. Miocamycin
2. Acecamycin
3. Miokamycin
4. Ponsinomycin
5. 3'',9-diacetylmydecamycin
6. 55881-07-7
7. Midecamycin Acetate [jan]
8. Midecamycin Diacetate
9. 9,3''-di-o-acetylmidecamycin
10. Leucomycin V, 3b,9-diacetate 3,4b-dipropanoate
11. 3t48cps7u2
12. Mom
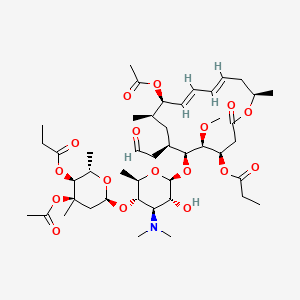
13. [(4r,5s,6s,7r,9r,10r,11e,13e,16r)-10-acetyloxy-6-[(2s,3r,4r,5s,6r)-5-[(2s,4r,5s,6s)-4-acetyloxy-4,6-dimethyl-5-propanoyloxyoxan-2-yl]oxy-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-5-methoxy-9,16-dimethyl-2-oxo-7-(2-oxoethyl)-1-oxacyclohexadeca-11,13-dien-4-yl] Propanoate
14. 9,3''-diacetylmidecamycin
15. Einecs 259-879-6
16. Unii-3t48cps7u2
17. Macroral
18. Miocamen
19. Mosil
20. Miocamycin (tn)
21. Miocamycin [mi]
22. Midecamycin Acetate (jp17)
23. Schembl139293
24. Chembl1091024
25. Dtxsid60905087
26. Leucomycin V, 3(sup B),9-diacetate 3,4(sup B)-dipropanoate
27. Midecamycin Acetate [who-dd]
28. Zinc169677000
29. 1532-rb
30. Leucomycinv,3b,9-diacetate3,4b-dipropanoate
31. D01636
32. Q3858659
33. W-105540
34. Midecamycin Acetate, Antibiotic For Culture Media Use Only
| Molecular Weight | 898.0 g/mol |
|---|---|
| Molecular Formula | C45H71NO17 |
| XLogP3 | 3.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 18 |
| Rotatable Bond Count | 18 |
| Exact Mass | 897.47219980 g/mol |
| Monoisotopic Mass | 897.47219980 g/mol |
| Topological Polar Surface Area | 218 Ų |
| Heavy Atom Count | 63 |
| Formal Charge | 0 |
| Complexity | 1590 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 16 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01F - Macrolides, lincosamides and streptogramins
J01FA - Macrolides
J01FA11 - Miocamycin