
1. Aureolic Acid
2. Mithramycin
3. Mitramycin
4. Plicamycin
1. Plicamycin
2. Mithramycin A
3. Aureolic Acid
4. 18378-89-7
5. Sk 26598
6. A-2371
7. Mithramycin A From Streptomyces Plicatus
8. Nsc24559
9. Pa-144
10. Nsc143020
11. Chembl509846
12. Hms2089f10
13. Rkl10094
14. Akos025311476
15. Ncgc00181781-01
16. Ab01275467-01
17. J-011777
18. Q26998354
19. Mithramycin A From Streptomyces Plicatus, >=90% (hplc)
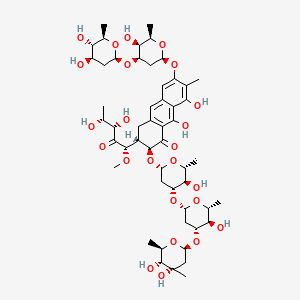
20. D-threo-2-pentulose,5-deoxy-1-c-[(2s,3s)-7-[[2,6-dideoxy-3-o-(2,6-dideoxy-b-d-arabino-hexopyranosyl)-b-d-arabino-hexopyranosyl]oxy]-3-[(o-2,6-dideoxy-3-c-methyl-b-d-ribo-hexopyranosyl-(1(r)3)-o-2,6-
| Molecular Weight | 1085.1 g/mol |
|---|---|
| Molecular Formula | C52H76O24 |
| XLogP3 | 0.6 |
| Hydrogen Bond Donor Count | 11 |
| Hydrogen Bond Acceptor Count | 24 |
| Rotatable Bond Count | 15 |
| Exact Mass | 1084.47265329 g/mol |
| Monoisotopic Mass | 1084.47265329 g/mol |
| Topological Polar Surface Area | 358 Ų |
| Heavy Atom Count | 76 |
| Formal Charge | 0 |
| Complexity | 1940 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 25 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Mesh Heading: Antibiotics, antineoplastic, fluorescent dyes, nucleic acid synthesis inhibitors, protein synthesis inhibitors
National Library of Medicine, SIS; ChemIDplus Record for Mithramycin (18378-89-7). Available from, as of April 17, 2006: https://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
Plicamycin is of limited value in the treatment of neoplastic disease because of its severe toxicity.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1247
Because of low incidence of testicular tumors (est. to be 1% of all tumors), number of cases evaluated is limited.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 1132
It has been beneficial in patients with disseminated testicular carcinomas, especially of the embryonal-cell type, but has been largely superseded by other drug regimens, particularly vinblastine, cisplatin, and bleomycin.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1247
For more Therapeutic Uses (Complete) data for MITHRAMYCIN (12 total), please visit the HSDB record page.
It is recommended that plicamycin be administered by intravenous infusion only to hospitalized patients by or under the supervision of a physician experienced in the use of cancer chemotherapeutic agents because of the possibility of severe reactions.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006.
Hemorrhagic syndrome occurs in about 5% of patients who receive no more than 30 ug/kg/day for no more than 10 doses, whereas it is about 12% for higher doses.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1082
BASELINE PLATELET COUNTS, PROTHROMBIN TIME, & BLEEDING TIME SHOULD BE DETERMINED BEFORE THERAPY IS BEGUN. THESE SHOULD BE MONITORED DURING THERAPY & FOR SEVERAL DAYS FOLLOWING LAST DOSE. SIGNIFICANT DECR IN PLATELET COUNT...OR SIGNIFICANT PROLONGATION OF PROTHROMBIN OR BLEEDING TIMES ARE INDICATIONS FOR DISCONTINUING DRUG.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 1133
METASTATIC LESIONS IN PATIENTS WITH MIXED PRIMARY TUMORS OF TESTES HAVE BEEN ONLY PARTIALLY REDUCED OR WERE UNAFFECTED... USUALLY, THESE...IDENTIFIED AS TERATOMAS, CHORIOCARCINOMAS, OR SEMINOMAS. USE...IS NOT RECOMMENDED FOR TREATMENT OF METASTASES DUE TO THESE CELL TYPES.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 1133
For more Drug Warnings (Complete) data for MITHRAMYCIN (17 total), please visit the HSDB record page.
Nucleic Acid Synthesis Inhibitors
Compounds that inhibit cell production of DNA or RNA. (See all compounds classified as Nucleic Acid Synthesis Inhibitors.)
Protein Synthesis Inhibitors
Compounds which inhibit the synthesis of proteins. They are usually ANTI-BACTERIAL AGENTS or toxins. Mechanism of the action of inhibition includes the interruption of peptide-chain elongation, the blocking the A site of ribosomes, the misreading of the genetic code or the prevention of the attachment of oligosaccharide side chains to glycoproteins. (See all compounds classified as Protein Synthesis Inhibitors.)
Calcium-Regulating Hormones and Agents
Hormones and molecules with calcium-regulating hormone-like actions that modulate OSTEOLYSIS and other extra-skeletal activities to maintain calcium homeostasis. (See all compounds classified as Calcium-Regulating Hormones and Agents.)
Fluorescent Dyes
Chemicals that emit light after excitation by light. The wave length of the emitted light is usually longer than that of the incident light. Fluorochromes are substances that cause fluorescence in other substances, i.e., dyes used to mark or label other compounds with fluorescent tags. (See all compounds classified as Fluorescent Dyes.)
Antibiotics, Antineoplastic
Chemical substances, produced by microorganisms, inhibiting or preventing the proliferation of neoplasms. (See all compounds classified as Antibiotics, Antineoplastic.)
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01D - Cytotoxic antibiotics and related substances
L01DC - Other cytotoxic antibiotics
L01DC02 - Plicamycin
It is not known whether plicamycin is excreted in breast milk.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006.
Duration of action: 7 to 10 days with a single dose.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006.
Time to peak effect: 72 hours with a single dose.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006.
Onset of action: When used for hypercalcemia, a reduction in plasma calcium usually occurs within 24 to 48 hours following administration.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006.
Plicamycin is concentrated in the Kupffer cells of the liver, in renal tubular cells, and along formed bone surfaces. It may localize in areas of active bone resorption. Plicamycin also crosses the blood-brain barrier and enters the cerebrospinal fluid (CSF).
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006.
The exact mechanism of action is unknown. However, it has been shown that plicamycin forms a complex with DNA in the presence of magnesium or other divalent cations, thereby inhibiting DNA-dependent or DNA-directed RNA synthesis.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006.
Plicamycin is believed to lower serum calcium concentrations, but the exact mechanism is unknown. It may act by blocking hypercalcemic action of vitamin D or by inhibiting the effect of parathyroid hormone on osteoclasts. Plicamycin's inhibition of DNA-dependent RNA synthesis appears to render osteoclasts unable to fully respond to parathyroid hormone with the biosynthesis necessary for osteolysis.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006.
The aureolic acid antitumor antibiotic mithramycin (MTM) inhibits both cancer growth and bone resorption by cross-linking GC-rich DNA, thus blocking binding of Sp-family transcription factors to gene regulatory elements. Transcription of c-src, a gene implicated in many human cancers and required for osteoclast-dependent bone resorption, is regulated by the binding of Sp factors to specific elements in its promoter. Therefore, this gene represents an important anticancer target and a potential lead target through which MTM displays its so far uncharacterized action against osteoclastic bone resorption. Here we demonstrate, using DNA binding studies, promoter reporter assays, and RT-PCR, that MTM inhibits Sp binding to the c-src promoter region, thereby decreasing its expression in human cancer cells. Furthermore, selected mithramycin analogues, namely, premithramycin B, mithramycin SK, 7-demethylmithramycin, 4E-ketomithramycin, and 4C-ketodemycarosylmithramycin, generated through combinatorial biosynthesis, were compared with MTM for their ability to block Sp binding to the c-src promoter. Although most of the tested compounds lost their ability to bind to the DNA, alteration of the MTM 3-pentyl side chain led to a compound (mithramycin SK) with the same DNA binding specificity but with lower binding affinity than MTM. While this compound was comparable to MTM in promoter reporter, gene expression, and anticancer assays, given its weaker interaction with the DNA, it may be much less toxic than MTM. The results presented here supplement recent findings and, moreover, allow new conclusions to be made regarding both the structure-activity relationships, particularly with respect to the alkyl side chains, and the mechanism of action of aureolic acid drugs.
PMID:12846580 Remsing LL, et al; Biochemistry 42 (27): 8313-24 (2003)
Mithramycin is an mRNA synthesis inhibitor that has been used to decrease bone resorption in patients with humoral hypercalcemia and Paget's disease. During studies on the mechanism of action of mithramycin it became clear that the compound has a direct inhibitory effect on osteoclastic bone resorption in the in vitro bone slice assay. At concentrations of 0.1 - 100 nM mithramycin directly inhibited osteoclastic bone resorption dose-dependently up to 66 + or - 5% at 100 nM (mean + or - SEM, 3 expts.). Another mRNA synthesis inhibitor, actinomycin D (0.1 - 100 nM) and the protein synthesis inhibitor, cycloheximide (0.1 - 10 M), also dose-dependently inhibited osteoclastic bone resorption by 78 + or - 7% at 100 nM and 76 + or - 7% at 10 M, respectively. Mithramycin and actinomycin D at 100 nM did not affect osteoclast survival on bone slices and were therefore not cytotoxic at the concentrations used. Mithramycin (100 nM) and cycloheximide (10 M) both slightly decreased osteoctast cytoplasmic spreading. Addition of 100 nM mithramycin 6 hr after osteoclast adhesion to bone slices still inhibited subsequent resorption by 50%, indicating a continued but lesser requirement for mRNA synthesis during bone resorption. These results show that 75% of osteoclasts obtained from neonatal rat long bones are activated by adhesion to mineralized bone surfaces and require mRNA and protein synthesis in order to resorb bone in vitro.
Hall TJ et al; J Biochemical and Biophysical Research Communications 195 (3): 1245-53 (1993)
For more Mechanism of Action (Complete) data for MITHRAMYCIN (6 total), please visit the HSDB record page.