


1. Bredinin
2. Mizoribine 5'-monophosphate
1. 50924-49-7
2. Bredinin
3. He-69
4. Mls000028813
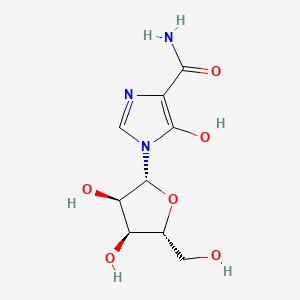
5. 1-((2r,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-hydroxy-1h-imidazole-4-carboxamide
6. He 69
7. 1-[(2r,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-hydroxyimidazole-4-carboxamide
8. Bredinine
9. 5-hydroxy-1-beta-d-ribofuranosyl-1h-imidazole-4-carboxamide
10. 50524-49-7
11. Smr000058473
12. 5-hydroxy-1-beta-d-ribofuranosylimidazole-4-carboxamide
13. 4jr41a10vp
14. 1h-imidazole-4-carboxamide, 5-hydroxy-1-beta-d-ribofuranosyl-
15. Nsc 289637
16. Nsc-289637
17. Ncgc00094087-01
18. Mizoribinum
19. Mizoribina
20. N'-(beta-d-ribofuranosyl)-5-hydroxyimidazole-4-carboxamide
21. Mizoribine [inn:jan]
22. Dsstox_cid_25777
23. Dsstox_rid_81119
24. Dsstox_gsid_45777
25. Mizoribinum [inn-latin]
26. Mizoribina [inn-spanish]
27. Bredinin (tn)
28. 1-[(2r,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-hydroxy-1h-imidazole-4-carboxamide
29. Cas-50924-49-7
30. Sr-01000075989
31. Brn 4151713
32. Unii-4jr41a10vp
33. 1h-imidazole-4-carboxamide, 5-hydroxy-1-.beta.-d-ribofuranosyl-
34. 4-carbamoyl-1-beta-d-ribofuranosyl-imidazolium-5-olate
35. Anhydro-4-carbamoyl-5-hydroxy-1-beta-d-ribofuranosyl-imidazolium Hydroxide
36. Mfcd00057221
37. Mizoribine(bredinin)
38. Hs-0046
39. Mizoribine (bredinin)
40. Mizoribine Hydrobromide
41. Mizoribine [mi]
42. Mizoribine [inn]
43. Mizoribine [jan]
44. Opera_id_1558
45. Spectrum2_001559
46. Spectrum3_000739
47. Spectrum4_000220
48. Spectrum5_001671
49. Mizoribine (jp17/inn)
50. M 3047
51. Mizoribine [mart.]
52. Schembl7118
53. Mizoribine [who-dd]
54. Lopac0_000745
55. Bspbio_002298
56. Kbiogr_000859
57. Mls001076272
58. Mls006010038
59. Divk1c_000948
60. Spbio_001438
61. Chembl245019
62. Cid_104762
63. Mizoribine, >=98% (tlc)
64. Dtxsid8045777
65. Bdbm68669
66. Chebi:31858
67. Hms502p10
68. Kbio1_000948
69. Kbio3_001518
70. Ninds_000948
71. Hms2230d04
72. Hms3262e12
73. 50924-49-7 (non-salt)
74. Ex-a2256
75. Zinc3812887
76. Tox21_111242
77. Tox21_500745
78. Ccg-39778
79. Fd9057
80. S1384
81. Akos015994615
82. Tox21_111242_1
83. Ac-5266
84. Bcp9000933
85. Cs-1823
86. Db12617
87. Lp00745
88. Sdccgsbi-0050723.p004
89. Idi1_000948
90. Smp1_000195
91. Ncgc00094087-02
92. Ncgc00094087-03
93. Ncgc00094087-04
94. Ncgc00094087-05
95. Ncgc00261430-01
96. Hy-17470
97. Bcp0726000318
98. Sbi-0050723.p003
99. Eu-0100745
100. M2399
101. A16794
102. D01392
103. Ab00053323_12
104. Ab00053323_13
105. 924m497
106. J-700185
107. Q6884708
108. Sr-01000075989-1
109. Sr-01000075989-4
110. 1-(beta-d-ribofuranosyl)-5-hydroxyimidazole-4-carboxamide
111. 4-carbamoyl-1-(beta-d-ribofuranosyl)-5-oxylatoimidazolium
112. 5-hydroxy-1-b-d-ribofuranosyl-1h-imidazole-4-carboxamide
113. 1h-imidazole-4-carboxamide, 5-hydroxy-1-b-d-ribofuranosyl-
114. 5-hydroxy-1-.beta.-d-ribofuranosylimidazole-4-carboxamide
115. 1-[(2r,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)-2-oxolanyl]-5-hydroxy-4-imidazolecarboxamide
116. 1-[(2r,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-5-hydroxy-imidazole-4-carboxamide
117. 1-[(2r,3r,4s,5r)-3,4-dihydroxy-5-methylol-tetrahydrofuran-2-yl]-5-hydroxy-imidazole-4-carboxamide
118. 1-[(2r,3r,4s,5r)-5-(hydroxymethyl)-3,4-bis(oxidanyl)oxolan-2-yl]-5-oxidanyl-imidazole-4-carboxamide
| Molecular Weight | 259.22 g/mol |
|---|---|
| Molecular Formula | C9H13N3O6 |
| XLogP3 | -1.9 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 3 |
| Exact Mass | 259.08043514 g/mol |
| Monoisotopic Mass | 259.08043514 g/mol |
| Topological Polar Surface Area | 151 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 329 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Antibiotics, Antineoplastic
Chemical substances, produced by microorganisms, inhibiting or preventing the proliferation of neoplasms. (See all compounds classified as Antibiotics, Antineoplastic.)
Antimalarials
Agents used in the treatment of malaria. They are usually classified on the basis of their action against plasmodia at different stages in their life cycle in the human. (From AMA, Drug Evaluations Annual, 1992, p1585) (See all compounds classified as Antimalarials.)
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Immunosuppressive Agents
Agents that suppress immune function by one of several mechanisms of action. Classical cytotoxic immunosuppressants act by inhibiting DNA synthesis. Others may act through activation of T-CELLS or by inhibiting the activation of HELPER CELLS. While immunosuppression has been brought about in the past primarily to prevent rejection of transplanted organs, new applications involving mediation of the effects of INTERLEUKINS and other CYTOKINES are emerging. (See all compounds classified as Immunosuppressive Agents.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)