

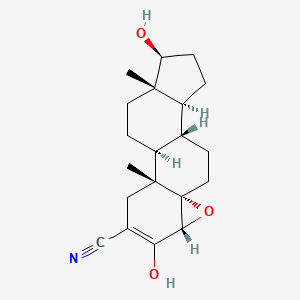

1. 4alpha,5-epoxy-17beta-hydroxy-3-oxoandrostane-2-carbonitrile
2. Modrenal
3. Win 24,540
1. 13647-35-3
2. Modrenal
3. Vetoryl
4. Desopan
5. Modrastane
6. Trilostanum
7. Trilostano
8. Win-24540
9. Win 24,540
10. Chebi:32260
11. L0fpv48q5r
12. Win 24540
13. Androst-2-ene-2-carbonitrile, 4,5-epoxy-3,17-dihydroxy-, (4alpha,5alpha,17beta)-
14. Nsc-758904
15. Dsstox_cid_3706
16. Androst-2-ene-2-carbonitrile, 4,5-epoxy-3,17-dihydroxy-, (4a,5a,17b)-
17. Dsstox_rid_77157
18. Dsstox_gsid_23706
19. (1ar,4ar,4bs,6as,7s,9as,9bs,11as)-2,7-dihydroxy-4a,6a-dimethyl-1a,4,4a,4b,5,6,6a,7,8,9,9a,9b,10,11-tetradecahydrocyclopenta[7,8]phenanthro[1,10a-b]oxirene-3-carbonitrile
20. (1s,2r,6r,8s,11s,12s,15s,16s)-5,15-dihydroxy-2,16-dimethyl-7-oxapentacyclo[9.7.0.0^{2,8}.0^{6,8}.0^{12,16}]octadec-4-ene-4-carbonitrile
21. Modrenal (tn)
22. (1ar,4ar,4bs,6as,7s,9as,9bs,11as)-2,7-dihydroxy-4a,6a-dimethyl-1a,4,4a,4b,5,6,6a,7,8,9,9a,9b,10,11-tetradecahydrocyclopenta[7,8]phenanthro[1,10a-b]oxirene-3-carbonitrile.
23. (1s,2r,6r,8s,11s,12s,15s,16s)-5,15-dihydroxy-2,16-dimethyl-7-oxapentacyclo[9.7.0.02,8.06,8.012,16]octadec-4-ene-4-carbonitrile
24. Desopan (tn)
25. Cas-13647-35-3
26. Trilostane (jan/usan)
27. Trilostanum [inn-latin]
28. Trilostano [inn-spanish]
29. Unii-l0fpv48q5r
30. Androst-2-ene-2-carbonitrile, 4,5-epoxy-3,17-dihydroxy-, (4.alpha.,5.alpha.,17.beta.)-
31. Trilostane [usan:inn:ban:jan]
32. Ncgc00274061-01
33. Einecs 237-133-0
34. Mfcd00199295
35. Win 24450
36. Trilostane [mi]
37. Trilostane [inn]
38. Trilostane [jan]
39. Trilostane [usan]
40. Trilostane [vandf]
41. (4alpha,5alpha,17beta)-3,17-dihydroxy-4,5-epoxyandrost-2-ene-2-carbonitrile
42. Schembl7517
43. Trilostane [mart.]
44. Trilostane [who-dd]
45. Gtpl6850
46. Trilostane [green Book]
47. Chembl1200907
48. Dtxsid9023706
49. Trilostane [orange Book]
50. Trilostane, >=98% (hplc)
51. 4.alpha.,5-epoxy-3,17.beta.-dihydroxy-5.alpha.-androst-2-ene-2-carbonitrile
52. Hms3884a15
53. Bcp04132
54. Tox21_112454
55. Tox21_302406
56. 4-alpha,5-epoxy-3,17-dihydroxy-5-alpha-androst-2-ene-2-carbonitrile
57. Ac-927
58. Bdbm50247882
59. S1404
60. 3,17beta-dihydroxy-4alpha,5-epoxy-5alpha-androst-2-ene-2-carbonitrile
61. 4alpha,5-epoxy-3,17beta-dihydroxy-5alpha-androst-2-ene-2-carbonitrile
62. Akos015964043
63. Akos032947221
64. Tox21_112454_1
65. Zinc100038546
66. Ccg-267803
67. Cs-1707
68. Db01108
69. Ks-1414
70. Nsc 758904
71. (2-alpha,4-alpha,5-alpha,17-beta)-4,5-epoxy-17-hydroxy-3-oxoandrostane-2-carbonitrile
72. 4-alpha-5-epoxy-17-beta-hydroxy-3-oxo-5-alpha-androstane-2-alpha-carbonitrile
73. Androstane-2-carbonitrile, 4,5-epoxy-17-hydroxy-3-oxo-, (2-alpha,4-alpha,5-alpha,17-beta)-
74. Ncgc00255201-01
75. Hy-14281
76. Bcp0726000098
77. D01180
78. Ab01274788-01
79. Ab01274788_02
80. 647t353
81. A886681
82. Q907313
83. Q-201884
84. Z1563146079
85. Trilostane; 13647-35-3; Vetoryl; Modrenal; Modrastane
86. (4a,5a,17b)-3,17-dihydroxy-4,5-epoxyandrost-2-ene-2-carbonitrile
87. 5-alpha-androstane-2-alpha-carbonitrile, 4-alpha,5-epoxy-17-beta-hydroxy-3-oxo-
| Molecular Weight | 329.4 g/mol |
|---|---|
| Molecular Formula | C20H27NO3 |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 329.19909372 g/mol |
| Monoisotopic Mass | 329.19909372 g/mol |
| Topological Polar Surface Area | 76.8 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 692 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used in the treatment of Cushing's syndrome. It is normally used in short-term treatment until permanent therapy is possible.
Trilostane blocks an enzyme involved in the production of several steroids including cortisol. Inhibiting this enzyme inhibits the production of cortisol. In Cushing's syndrome, the adrenal gland overproduces steroids. Although steroids are important for various functions of the body, too much can cause problems. Trilostane reduces the amount of steroids produced by the adrenal gland. This product was withdrawn from the U.S. market in April 1994.
Abortifacient Agents, Steroidal
Steroidal compounds with abortifacient activity. (See all compounds classified as Abortifacient Agents, Steroidal.)
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
H - Systemic hormonal preparations, excl. sex hormones and insulins
H02 - Corticosteroids for systemic use
H02C - Antiadrenal preparations
H02CA - Anticorticosteroids
H02CA01 - Trilostane
Hepatic.
8 hours.
Trilostane produces suppression of the adrenal cortex by inhibiting enzymatic conversion of steroids by 3-beta-hydroxysteroid dehydrogenase/delta 5,4 ketosteroid isomerase, thus blocking synthesis of adrenal steroids.