


1. En 313
2. En-313
3. En313
4. Ethmozin
5. Ethmozine
6. Etmozin
7. Hydrochloride, Moricizine
8. Moracizin
9. Moracizine
10. Moricizine
1. 29560-58-5
2. Moracizine Hydrochloride
3. Ethmozine
4. Ethmosine
5. Moricizine Hcl
6. Moricizine (hydrochloride)
7. Ethmozin
8. Etmozin
9. 71ok3z1esp
10. Mls000830274
11. Moricizine Hydrochloride [usp]
12. En 313; Ethmosine; Ethmozin
13. En 313
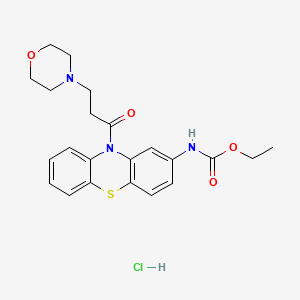
14. Ethyl N-[10-(3-morpholin-4-ylpropanoyl)phenothiazin-2-yl]carbamate;hydrochloride
15. Moracizine Hcl
16. Smr000036736
17. Ethyl 10-(3-morpholinopropionyl)phenothiazine-2-carbamate, Hydrochloride
18. Dsstox_cid_27766
19. Dsstox_rid_82543
20. Dsstox_gsid_47786
21. Carbamic Acid, (10-(3-(4-morpholinyl)-l-oxopropyl)-10h-phenothiazin-2yl)-, Ethyl Ester, Hydrochloride
22. Moricizine Hydrochloride (usp)
23. Ethyl 10-(beta-n-morpholinylpropionyl)phenothiazine-2-carbamate Hydrochloride
24. Chebi:60937
25. Cas-29560-58-5
26. Ncgc00016809-01
27. Ncgc00016809-07
28. Unii-71ok3z1esp
29. Ethmozine (tn)
30. Opera_id_267
31. Ethyl 10-(3-morpholinopropionyl)phenothiazine-2-carbamate Hydrochloride
32. Cambridge Id 5229972
33. Ethyl Ether Of 10-(beta-morpholylpropionyl)phenthiazinecarbamino Acid Hydrochloride
34. Moracizinehydrochloride
35. Schembl41358
36. (10-(3-(4-morpholinyl)-1-oxopropyl)-10h-phenothiazin-2-yl)carbamic Acid, Ethyl Ester, Monohydrochloride
37. Mls000080120
38. Regid_for_cid_34632
39. Chembl1200334
40. Dtxsid1047786
41. Hy-b0615a
42. Hms1571c04
43. Ethyl 10-(3-morpholinopropanoyl)-
44. Tox21_110622
45. Moricizine Hydrochloride [mi]
46. Akos030506898
47. Tox21_110622_1
48. Ccg-221051
49. Moracizine Hydrochloride [mart.]
50. Moricizine Hydrochloride [vandf]
51. Phenothiazine-2-carbamic Acid, 10-(3-morpholinopropionyl)-, Ethyl Ester, Hydrochloride
52. Moracizine Hydrochloride [who-dd]
53. Moricizine Hydrochloride [usp-rs]
54. Ncgc00180924-01
55. Carbamic Acid, (10-(3-(4-morpholinyl)-1-oxopropyl)-10h-phenothiazin-2-yl)-, Ethyl Ester, Monohydrochloride
56. Db-047605
57. Cs-0013699
58. E-313
59. E-350
60. Ft-0630550
61. Moricizine Hydrochloride [orange Book]
62. D02087
63. Moricizine Hydrochloride [usp Impurity]
64. Sr-01000614327
65. Sr-01000614327-4
66. Q27130132
67. Ethyl N-[10-(3-morpholin-4-ylpropanoyl)phenothiazin-2-yl]carbamate;hydron;chloride
68. Carbamic Acid, N-(10-(3-(4-morpholinyl)-1-oxopropyl)-10h-phenothiazin-2-yl)-, Ethyl Ester, Hydrochloride (1:1)
| Molecular Weight | 464.0 g/mol |
|---|---|
| Molecular Formula | C22H26ClN3O4S |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Exact Mass | 463.1332552 g/mol |
| Monoisotopic Mass | 463.1332552 g/mol |
| Topological Polar Surface Area | 96.4 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 601 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Anti-Arrhythmia Agents
Agents used for the treatment or prevention of cardiac arrhythmias. They may affect the polarization-repolarization phase of the action potential, its excitability or refractoriness, or impulse conduction or membrane responsiveness within cardiac fibers. Anti-arrhythmia agents are often classed into four main groups according to their mechanism of action: sodium channel blockade, beta-adrenergic blockade, repolarization prolongation, or calcium channel blockade. (See all compounds classified as Anti-Arrhythmia Agents.)
Voltage-Gated Sodium Channel Blockers
A class of drugs that inhibit the activation of VOLTAGE-GATED SODIUM CHANNELS. (See all compounds classified as Voltage-Gated Sodium Channel Blockers.)

LOOKING FOR A SUPPLIER?