


1. Cellcept
2. Mofetil Hydrochloride, Mycophenolate
3. Mofetil, Mycophenolate
4. Mycophenolate Mofetil
5. Mycophenolate Mofetil Hydrochloride
6. Mycophenolate, Sodium
7. Mycophenolic Acid
8. Mycophenolic Acid Morpholinoethyl Ester
9. Myfortic
10. Rs 61443
11. Rs-61443
12. Rs61443
13. Sodium Mycophenolate
1. 37415-62-6
2. Sodium Mycophenolate
3. Mycophenolic Acid Sodium Salt
4. Mycophenolic Acid Monosodium Salt
5. Erl 080
6. Mycophenolate Sodium [usan]
7. Sodium Mycophenalate
8. Erl-080
9. Mycophenolic Acid, Sodium Salt
10. Wx877sqi1g
11. Chebi:67155
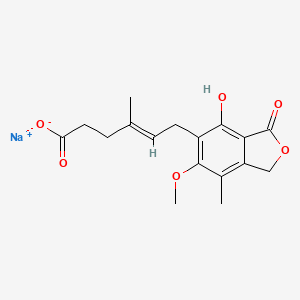
12. Sodium;(e)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1h-2-benzofuran-5-yl)-4-methylhex-4-enoate
13. Nsc-116072
14. 4-hexenoic Acid, 6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-, Sodium Salt(1:1) , (4e)-
15. Mycophenolic Acid (sodium)
16. 4-hexenoic Acid, 6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-, Monosodium Salt, (4e)-
17. Sodium 4(e)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihydroisobenzofuran-5-yl)-4-methylhex-4-enoate
18. Sodium (e)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihydroisobenzofuran-5-yl)-4-methylhex-4-enoate
19. Unii-wx877sqi1g
20. Nsc 116072
21. Femulan
22. Erl 080a
23. Mycophenolic Sod
24. Myfortic (tn)
25. Ec-mps
26. Starbld0043375
27. Mycophenolatesodium
28. Mycophenolate Sodium (usp)
29. 4-hexenoic Acid, 6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-, Monosodium Salt, (e)-
30. Erl-080a
31. Schembl1649229
32. Chembl2106643
33. Hy-b0421a
34. Mycophenolate Sodium [vandf]
35. Mycophenolate Sodium [mart.]
36. Mycophenolate Sodium [usp-rs]
37. Mycophenolate Sodium [who-dd]
38. Akos015969286
39. 4-hexenoic Acid, 6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-5-phthalanyl)-4-methyl-, Sodium Salt
40. As-17786
41. Mycophenolate Sodium [ep Monograph]
42. Mycophenolic Acid Sodium Salt [mi]
43. Mycophenolate Sodium [usp Monograph]
44. Cs-0103129
45. D05095
46. D93087
47. Q27135653
48. Sodium (4e)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihydro-2-benzofuran-5-yl)-4-methylhex-4-enoate
49. Sodium;(e)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1h-2-benzofuran-5-yl)-4-methylhex-4-enoic Acid
| Molecular Weight | 342.3 g/mol |
|---|---|
| Molecular Formula | C17H19NaO6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Exact Mass | 342.10793260 g/mol |
| Monoisotopic Mass | 342.10793260 g/mol |
| Topological Polar Surface Area | 95.9 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 492 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Antibiotics, Antineoplastic
Chemical substances, produced by microorganisms, inhibiting or preventing the proliferation of neoplasms. (See all compounds classified as Antibiotics, Antineoplastic.)
Antibiotics, Antitubercular
Substances obtained from various species of microorganisms that are, alone or in combination with other agents, of use in treating various forms of tuberculosis; most of these agents are merely bacteriostatic, induce resistance in the organisms, and may be toxic. (See all compounds classified as Antibiotics, Antitubercular.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)