
1. Dimethylnitrosamine
2. N Nitrosodimethylamine
3. Ndma Nitrosodimethylamine
4. Nitrosodimethylamine
5. Nitrosodimethylamine, Ndma
1. Dimethylnitrosamine
2. 62-75-9
3. Ndma
4. Dimethylnitrosoamine
5. N-methyl-n-nitrosomethanamine
6. N,n-dimethylnitrous Amide
7. N,n-dimethylnitrosamine
8. Dmna
9. Methanamine, N-methyl-n-nitroso-
10. Nitrosodimethylamine
11. Dimethylnitrosamin
12. N-nitroso-n,n-dimethylamine
13. Dimethylamine, N-nitroso-
14. N-nitroaodimethylamine
15. N-dimethyl-nitrosamine
16. Rcra Waste Number P082
17. Nitrous Dimethylamide
18. Dmn
19. Nsc 23226
20. N-methyl-n-nitroso-methanamine
21. Dimethylnitrosamin [german]
22. 1,1-dimethyl-2-oxohydrazine
23. N-dimethylnitrosoamine
24. Dimethyl(nitroso)amine
25. N-nitroso-dimethylamine
26. (ch3)2nno
27. Mls001065602
28. M43h21io8r
29. Chebi:35807
30. Nsc-23226
31. Smr000568479
32. Dsstox_cid_1029
33. Dimethylnitrosamin (german)
34. Dsstox_rid_75914
35. Dsstox_gsid_21029
36. Nitrosamine, Dimethyl-
37. Cas-62-75-9
38. Ccris 261
39. N Nitrosodimethylamine
40. Methanamine,n-methyl-n-nitroso-
41. Hsdb 1667
42. Ndma Nitrosodimethylamine
43. Einecs 200-549-8
44. Nitrosodimethylamine, Ndma
45. Rcra Waste No. P082
46. Brn 1738979
47. Unii-m43h21io8r
48. N-nitrosodimethylamine (ndma)
49. Ai3-25308
50. Methamine, N-methyl-n-nitroso-
51. Dimethyl-nitrosamine
52. N, N-dimethylnitrosamine
53. Nmda (genotoxic)
54. N,n-dimethyl Nitrous Amide
55. N,n-dimethyl-nitrous Amide
56. Cid_6124
57. N, N-dimethyl Nitrous Amide
58. N-n-dimethyl N Nitrosoamine
59. N-methyl-n-nitroso-methamine
60. Bidd:er0584
61. Wln: Onn1&1
62. Chembl117311
63. Dtxsid7021029
64. 1,1-dimethyl-2-oxohydrazine #
65. Bdbm73982
66. N-nitrosodimethylamine [mi]
67. Nsc23226
68. Zinc4658628
69. N-methyl-n-nitrosomethanamine, 9ci
70. Tox21_201663
71. Tox21_302873
72. N-nitrosodimethylamine [hsdb]
73. N-nitrosodimethylamine [iarc]
74. Akos016000499
75. At21751
76. N-nitrosodimethylamine [usp-rs]
77. Ncgc00091431-01
78. Ncgc00091431-02
79. Ncgc00091431-03
80. Ncgc00256498-01
81. Ncgc00259212-01
82. N-nitrosodimethylamine, Analytical Standard
83. D0761
84. Ft-0672951
85. N-nitrosodimethylamine 0.2 Mg/ml In Methanol
86. A833977
87. N-nitroso-dimethylamine 10 Microg/ml In Methanol
88. N-nitroso-dimethylamine 100 Microg/ml In Methanol
89. N-nitrosodimethylamine 1000 Microg/ml In Methanol
90. Q409367
91. N-nitrosodimethylamine 1000 Microg/ml In Dichloromethane
92. N-nitrosodimethylamine (ndma) 5000 Microg/ml In Methanol
93. N-nitrosodimethylamine 1000 Microg/ml In Methanol, Second Source
| Molecular Weight | 74.08 g/mol |
|---|---|
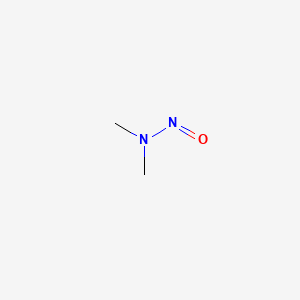
| Molecular Formula | C2H6N2O |
| XLogP3 | -0.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 74.048012819 g/mol |
| Monoisotopic Mass | 74.048012819 g/mol |
| Topological Polar Surface Area | 32.7 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 34.6 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
In order to elicit the characteristic toxicities, DMNA has required activation to a proximate mutagenic and carcinogenic metabolite. DMNA has undergone oxidative demethylation as catalyzed by rodent and human hepatic microsomes; this reaction has given rise to reactive intermediates. Alkylation of guanine (to 7-methylguanlic acid) and of cytosine (to 3-methylcytosine) has been thought to underlie the biochemical basis of DMNA-induced carcinogenesis. Methylation of the messenger ribonucleic acid (mRNA) in DMNA poisoning inhibited transIation. Although 7- methylguanylic acid has served as a normal nucleotide for RNA polymerase activity, the incorporation of 3-methylcytosine reduced efficiency. The alkylation of DNA by the reactive metabolites of DMNA resulted in a change in base sequence and deletion of one or more base pairs, changes indicative of the genotoxic mechanism of DMNA-induced carcinogenesis.
American Conference of Governmental Industrial Hygienists. Documentation of the TLV's and BEI's with Other World Wide Occupational Exposure Values. CD-ROM Cincinnati, OH 45240-4148 2010.
It is absorbed from gastrointestinal tract and lung ... Skin absorption is slow. When administered to rats, mice, and rabbits, it is distributed uniformly in tissue ... Although the liver is main organ concerned with its metabolism and is site of selective toxicity, dimethylnitrosamine does not concentrate there. Only a small percentage is excreted unchanged in rat urine after oral and iv doses of 50 to 100 mg/kg. A large proportion ... Appears in the expired air as ... Carbon dioxide (approx 60% in 24 hr).
Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 2787
N-nitrosodimethylamine (NDMA) reached equal max concn in the maternal and fetal blood and tissues within 5-15 min and 30-60 min, respectively, after an iv injection of 50 mg/kg into mice and rats on days 21-23 of pregnancy. The subsequent exponential decr in NDMA was more rapid in mice than in rats. Increasing the NDMA level in maternal blood by increased doses, linearly increased the fetal blood NDMA to a peak of 120 mug/mL in mice and 220 ug/mL in rats, whereas the level of NDMA in maternal blood continued to increase as doses were increased to 300 mg/kg.
Shendrikova IA et al; Farmakol Toksikol (Moscow) 46 (6): 53-7 (1983)
N-Nitrosodimethylamine (DMN) was shown to be assimilated by the roots and translocated to the tops of lettuce and spinach plants.
Dean-Raymond D, Alexander M; Nature 262: 394-6 (1976)
The acute toxicities of dimethylnitrosamine and diethylnitrosamine were evaluated in adult male crayfish. Toxicokinetic studies of (14)C dimethylnitrosamine and (14)C diethylnitrosamine in Austropotamobius pallipes (crayfish), administered by iv injection, show high concns of (14)C in abdominal muscle and hepatopancreas. Excretion is greater with dimethylnitrosamine, and retention in tissues, especially the hepatopancreas, is greater with diethylnitrosamine.
PMID:4090529 Alibaud R et al; Xenobiotica 15 (12): 1103-10 (1985)
Formation of n-nitrosodimethylamine (NDMA) in the stomachs of rats and mice after simultaneous oral administration of (14)C-labeled dimethylamine and potassium nitrite was determined by measuring the methylation of liver DNA. Simultaneous administration of 50 mg ascorbate/kg inhibited the nitrosation by approximately 80%. 50 mg alpha-tocopherol acetate/kg reduced the nitrosation by approximately 50%.
PMID:6683225 Meier-Bratschi A et al; Food Chem Toxicol 21 (3): 285-9 (1983)
A method for quantitative estimation of the formation of N-nitrosodimethylamine (NDMA) in mice was developed. When 0.25 umole of aminopyrine and 0.25-2.0 umole of sodium nitrite were simultaneously administration orally to mice, the amt of NDMA formed in 20 min was 8.2-60.3 nmol. These values are equal to approximately 30-200 ug/kg of body wt which are nearly daily doses expected to cause carcinogenic effect in mice or rats.
PMID:6667123 Kawanishi T et al; Arch Toxicol 54 (4): 323-30 (1983)
Available evidence suggests that NDMA requires metabolic activation to exert its toxic and carcinogenic effects. Rate of metabolism ... in vivo has been exam by measuring rate of loss of NDMA from blood and exhalation of (14)CO2 following administration of (14)C-NDMA. In rats ... 30 mg/kg administration by ip injection is metabolized within 6 hr. Rate of metab of NDMA in vitro ... Measured by use of slices of liver and other organs ... from rats, hamsters, monkeys, trout, goldfish and various amphibians. ... Oxidative N-demethylation to form formaldehyde has been demonstrated with liver microsomes from rats, mice and hamsters. Microsomal oxidn has been suggested to result in unstable N-nitroso-n-methyl-n-hydroxymethylamine, which decomp to yield methylating species and formaldehyde. ...
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V17 146 (1978)
... In the metabolism ... one of the intermediary products is diazomethane.
International Labour Office. Encyclopedia of Occupational Health and Safety. Vols. I&II. Geneva, Switzerland: International Labour Office, 1983., p. 621
For more Metabolism/Metabolites (Complete) data for N-NITROSODIMETHYLAMINE (16 total), please visit the HSDB record page.
N-Nitrosodimethylamine has known human metabolites that include N-Nitroso-N-methyl-N-hydroxymethylamine, N-Nitrosomethylamine, and acetyl-(dimethylamino)-oxoazanium.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
No reports found; [TDR, p. 955]
TDR - Ryan RP, Terry CE, Leffingwell SS (eds). Toxicology Desk Reference: The Toxic Exposure and Medical Monitoring Index, 5th Ed. Washington DC: Taylor & Francis, 1999., p. 955
When administered to rats, mice and rabbits, it is ... metabolized rapidly, with a half-life of approximately 4 hr.
Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 2787
The effect of dimethylnitrosamine on functional activities of liver chromatin was studied in mice. After a single dose of dimethylnitrosamine injected i.v. (25 mg/kg body wt, 45 min before sacrifice) liver nuclei were isolated and incubated with micrococcal nuclease (EC 3.1.4.7) to an acid-solubility of 2.5% of total DNA. Chromatin was fractionated into a 1,200 g pellet P1, 102,000 g pellet P2 and supernatant fraction S2. Chromatin-bound RNA polymerase I plus III activity decreased 15% in the P1 and 25% in the P2 fraction. No changes in activity were observed in the S2 fraction. Chromatin-bound RNA polymerase II activity decreased 19% in the P1, 49% in the P2 and 32% in the S2 fraction. Heparin stimulated RNA polymerase II activity decreased 10% in the P1 and 44% in the P2 fraction. Formation of initiation in nuclear lysates with RNA polymerase from Escherichia coli increased after administration of dimethylnitrosamine suggesting an increase in the number of sites available for the start of new RNA chains. The results show that limited digestion of nuclei with endonuclease cleaves chromatin regions which are more affected by dimethylnitrosamine than the total chromatin suggesting a non-random effect of the hepatotoxin on chromatin. Modifications of the DNA template by dimethylnitrosamine is indicated by the change in number of initiation complexes.
PMID:6197951 Klaude M, Von Der Decken A; Arch Toxicol 54 (3): 215-25 (1983)
... Hamsters are more susceptible than rats to liver cancer by dimethylnitrosamine because of its deficiency in repair of O6-alkylation of guanine in DNA. In rats a single large dose of dimethylnitrosamine induces kidney, but not liver, cancer because of the capability of liver repair enzymes to remove alkylated forms of DNA from the liver.
Amdur, M.O., J. Doull, C.D. Klaasen (eds). Casarett and Doull's Toxicology. 4th ed. New York, NY: Pergamon Press, 1991., p. 148