
1. Nafcil
2. Nafcillin Sodium
3. Nafcillin, Monosodium Salt, Anhydrous
4. Nafcillin, Sodium
5. Naphthamidopenicillin
6. Sodium Nafcillin
7. Sodium, Nafcillin
1. Naphcillin
2. Nallpen
3. 147-52-4
4. Nafcilina
5. Nafcilline
6. Nafcillinum
7. Unipen
8. (2-ethoxy-1-naphthyl)penicillin
9. Nafcilina [inn-spanish]
10. Nafcilline [inn-french]
11. Nafcillinum [inn-latin]
12. Nafcillin Sodium Salt
13. (2-ethoxy-1-naphthalenyl)penicillin
14. Chebi:7447
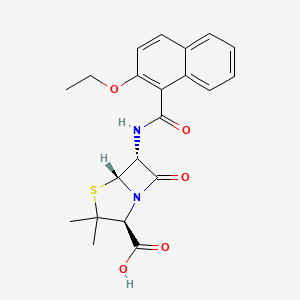
15. (2s,5r,6r)-6-[(2-ethoxynaphthalene-1-carbonyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid
16. Nafcillin (inn)
17. Penicillin, (2-ethoxy-1-naphthalenyl)-
18. 6-(2-ethoxy-1-naphthamido)penicillanic Acid
19. 4cnz27m7rv
20. Nafcilin-1
21. Nafcillin [inn]
22. Nafcillin [inn:ban]
23. (2s,5r,6r)-6-[(2-ethoxy-1-naphthoyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid
24. (2s,5r,6r)-6-{[(2-ethoxynaphthalen-1-yl)carbonyl]amino}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid
25. 4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid, 6-[[(2-ethoxy-1-naphthalenyl)carbonyl]amino]-3,3-dimethyl-7-oxo-, (2s,5r,6r)-
26. Hsdb 3133
27. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 6-(2-ethoxy-1-naphthamido)-3,3-dimethyl-7-oxo-
28. Einecs 205-690-9
29. Penicillin, (2-ethoxy-1-naphthyl)-
30. Unii-4cnz27m7rv
31. Brn 0862393
32. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 6-(((2-ethoxy-1-naphthalenyl)carbonyl)amino)-3,3-dimethyl-7-oxo-, (2s,5r,6r)-
33. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 6-(((2-ethoxy-1-naphthalenyl)carbonyl)amino)-3,3-dimethyl-7-oxo-, (2s-(2alpha,5alpha,6beta))-
34. Nafcillin, Antibiotic For Culture Media Use Only
35. Nafcillin [mi]
36. Nafcillin [hsdb]
37. Prestwick0_000843
38. Prestwick1_000843
39. Prestwick2_000843
40. Prestwick3_000843
41. Nafcillin [vandf]
42. Epitope Id:141583
43. Nafcillin [who-dd]
44. Chembl1443
45. Schembl47797
46. Bspbio_000825
47. Spbio_002746
48. Bpbio1_000909
49. Dtxsid8023343
50. Gtpl10942
51. Ethoxynaphthamido Penicillin Sodium
52. Bcp17447
53. Hy-b0555
54. Zinc3875980
55. Bdbm50103525
56. Db00607
57. 6beta-(2-ethoxynaphthalene-1-carboxamido)-2,2-dimethylpenam-3alpha-carboxylic Acid
58. (2s,5r,6r)-6-({[2-(ethyloxy)naphthalen-1-yl]carbonyl}amino)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid
59. (2s,5r,6r)-6-(2-ethoxynaphthalene-1-amido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid
60. Cs-0009511
61. C07250
62. D08242
63. 177n506
64. Q1638852
65. W-108115
66. Brd-k18574842-323-03-3
67. (2s,5r,6r)-6-(2-ethoxy-1-naphthamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid
68. (2s-(2.alpha.,5.alpha.,6.beta.))-6-(((2-ethoxy-l-naphthalenyl)carbonyl)-amino)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid
| Molecular Weight | 414.5 g/mol |
|---|---|
| Molecular Formula | C21H22N2O5S |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 414.12494298 g/mol |
| Monoisotopic Mass | 414.12494298 g/mol |
| Topological Polar Surface Area | 121 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 699 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Nafcillin sodium |
| Drug Label | Nafcillin for Injection, USP is a semisynthetic antibiotic substance derived from 6-amino-penicillanic acid. It is the sodium salt in a parenteral dosage form. The chemical name of nafcillin sodium is monosodium (2S,5R,6R)-6-(2-ethoxy-1-naphthamido)-... |
| Active Ingredient | Nafcillin sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 2gm base/vial; eq 10gm base/vial; eq 1gm base/vial |
| Market Status | Prescription |
| Company | Istituto Bio Ita Spa; Aurobindo Pharma; Sandoz; Antibiotice; Sagent Pharms; Agila Speclts |
| 2 of 4 | |
|---|---|
| Drug Name | Nallpen in plastic container |
| Active Ingredient | Nafcillin sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 2gm base/100ml; eq 20mg base/ml |
| Market Status | Prescription |
| Company | Baxter Hlthcare |
| 3 of 4 | |
|---|---|
| Drug Name | Nafcillin sodium |
| Drug Label | Nafcillin for Injection, USP is a semisynthetic antibiotic substance derived from 6-amino-penicillanic acid. It is the sodium salt in a parenteral dosage form. The chemical name of nafcillin sodium is monosodium (2S,5R,6R)-6-(2-ethoxy-1-naphthamido)-... |
| Active Ingredient | Nafcillin sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 2gm base/vial; eq 10gm base/vial; eq 1gm base/vial |
| Market Status | Prescription |
| Company | Istituto Bio Ita Spa; Aurobindo Pharma; Sandoz; Antibiotice; Sagent Pharms; Agila Speclts |
| 4 of 4 | |
|---|---|
| Drug Name | Nallpen in plastic container |
| Active Ingredient | Nafcillin sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 2gm base/100ml; eq 20mg base/ml |
| Market Status | Prescription |
| Company | Baxter Hlthcare |
Penicillins
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
NAFCILLIN IS SIMILAR TO OXACILLIN IN ITS POTENCY AGAINST /PENICILLINASE-PRODUCING/ PENICILLIN G-RESISTANT STAPH AUREUS, BUT NOT AS ACTIVE AS PENICILLIN G AGAINST STAPHYLOCOCCI SENSITIVE TO THE LATTER AGENT. NAFCILLIN IS INACTIVATED TO A VARIABLE DEGREE IN ACIDIC MEDIUM OF GASTRIC CONTENTS.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1078
ALTHOUGH...ALSO EFFECTIVE AGAINST NON-PENICILLINASE-PRODUCING STREPTOCOCCI, PNEUMOCOCCI, & GONOCOCCI, IT IS USUALLY NOT USED TO TREAT INFECTIONS CAUSED BY THESE BACTERIA UNLESS THEY ARE PART OF A MIXED INFECTION WITH PENICILLIN G-RESISTANT STAPHYLOCOCCI. /NAFCILLIN SODIUM/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1130
NAFCILLIN...SUPPRESSES GROWTH OF E COLI & CERTAIN OTHER ORGANISMS THAT CAUSE URINARY TRACT INFECTIONS, &...IS SOMETIMES USED TO TREAT SUCH INFECTIONS; HOWEVER, AFTER ORAL ADMIN, ESP, URINE LEVELS OF DRUG ARE GENERALLY LOWER THAN WITH...OTHER PENICILLINS OR TOO ERRATIC, SO PARENTERAL ADMIN OF OTHER PENICILLINS...PREFERRED.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1130
For more Therapeutic Uses (Complete) data for NAFCILLIN (14 total), please visit the HSDB record page.
3 PEDIATRIC PATIENTS ON 100-180 MG/KG/DAY NAFCILLIN DEVELOPED NEUTROPENIA. RECOMMENDED THAT ABSOLUTE NEUTROPHIL COUNT LESS THAN 1000/ML INDICATES CHANGE TO NON-PENICILLIN ANTIBIOTIC. CHILDREN ON IV PENICILLINS SHOULD HAVE WBC COUNTS WITH DIFFERENTIAL ANALYSIS 2-3 TIMES/WK.
PMID:263880 GREENE GR, E COHEN; PEDIATRICS 61: 94-7 (1978)
UNUSUALLY HIGH OCCURRENCE OF DRUG REACTIONS WITH NAFCILLIN.
PMID:155936 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2595628 ZAKHIREH B, RK ROOT; YALE J BIOL MED 51 (4): 449-55 (1978)
Because penicillins are distributed into milk, nafcillin should be used with caution in nursing women.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 95. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1995 (Plus Supplements 1995)., p. 271
Penicillins are distributed into breast milk, some in low concentrations. Although significant problems in humans have not been documented, the use of penicillins by nursing mothers may lead to sensitization, diarrhea, candidiasis, and skin rash in the infant. /Penicillins/
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 2151
For more Drug Warnings (Complete) data for NAFCILLIN (17 total), please visit the HSDB record page.
Indicated in the treatment of infections caused by penicillinase-producing staphylococci which have demonstrated susceptibility to the drug.
FDA Label
Nafcillin is a semisynthetic antibiotic substance derived from 6-amino-penicillanic acid. The drugs in this class are highly resistant to inactivation by staphylococcal penicillinase and are active against penicillinase-producing and non penicillinase-producing strains of Staphylococcus species.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01C - Beta-lactam antibacterials, penicillins
J01CF - Beta-lactamase resistant penicillins
J01CF06 - Nafcillin
Absorption
Following intravenous administration of 500mg nafcillin, the mean plasma concentration was approximately 30 g/mL. This value was reached after 5 minutes of injection.
Route of Elimination
Nafcillin is primarily eliminated by non-renal routes, namely hepatic inactivation and excretion in the bile.
Volume of Distribution
Nafcillin is reported to be widely distributed in various body fluids, including bile, pleural, amniotic and synovial fluids.
...ABSORPTION AFTER ORAL ADMIN IS IRREGULAR...WHETHER...TAKEN WITH MEALS OR ON EMPTY STOMACH. AFTER PARENTERAL ADMIN, PLASMA CONCN OF NAFCILLIN IS LOWER THAN...EQUIV DOSE OXACILLIN. THIS IS ASSOC WITH...LARGER APPARENT VOL DISTRIBUTION OF NAFCILLIN, RESULTING FROM SELECTIVE SEQUESTRATION...IN LIVER & POSSIBLY OTHER TISSUE
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1078
...BOUND TO PLASMA PROTEIN TO EXTENT OF ABOUT 90%. ONLY APPROX 10% OF ORAL DOSE...RECOVERABLE IN URINE. PROBENECID FURTHER REDUCES EXCRETION IN URINE. MAJOR CHANNEL OF ELIMINATION...IS BILE...ABOUT 90% OF SINGLE IV DOSE... ACCOUNTED FOR BY BILIARY EXCRETION; THERE IS SOME REABSORPTION OF DRUG FROM SMALL INTESTINE.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1078
PEAK CONCN OF NAFCILLIN IN BILE ARE WELL ABOVE THOSE FOUND IN PLASMA.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1078
A 1 G ORAL DOSE NAFCILLIN SODIUM REACHED BLOOD CONCN 0.7 MG% @ 1 HR; 0.5 G IM DOSE REACHED CONCN 0.6 MG% @ 1 HR. 17% OF AN ORAL DOSE WAS EXCRETED IN URINE; 36% OF IM DOSE WAS EXCRETED IN URINE. /NAFCILLIN SODIUM/
Sunshine, I. (ed.). CRC Handbook of Analytical Toxicology. Cleveland: The Chemical Rubber Co., 1969., p. 364
For more Absorption, Distribution and Excretion (Complete) data for NAFCILLIN (19 total), please visit the HSDB record page.
Hepatic metabolism accounts for less than 30% of the biotransformation of most penicillins.
...PROTON-CATALYZED HYDROLYSIS /OF PENICILLINS/ TO YIELD PENICILLOIC ACIDS IS WELL DOCUMENTED...CATALYZED BY BACTERIAL BETA-LACTAMASE & BY MAMMALIAN ENZYMES. /PENICILLINS/
Testa, B. and P. Jenner. Drug Metabolism: Chemical & Biochemical Aspects. New York: Marcel Dekker, Inc., 1976., p. 151
NAFCILLIN GIVES 2-ETHOXY-1-NAPHTHAMIDOPENICILLOIC ACID IN BACILLUS. /FROM TABLE/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. E-7
Approx 60% of a dose of nafcillin is metabolized in the liver to inactive metabolites.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 95. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1995 (Plus Supplements 1995)., p. 271
The serum half-life of nafcillin administered by the intravenous route ranged from 33 to 61 minutes as measured in three separate studies.
NORMAL T1/2 OF NAFCILLIN IS 0.5 HR. /FROM TABLE/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1078
The serum half-life of nafcillin in adults with normal renal and hepatic function averages 0.5-1.5 hr. In one study in healthy adults, nafcillin had a distribution half-life ... of 0.17 hr and an elimination half-life ... of 1.02 hr.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 95. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1995 (Plus Supplements 1995)., p. 271
The serum half-life of ... /nafcillin/ is reportedly 1.2-1.9 hr in patients with creatinine clearances of 3-59 ml/min per 1.73 sq meters and 1.8-2.8 hr in patients with creatinine clearances less than 3 ml/min per 1.73 sq meters.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 95. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1995 (Plus Supplements 1995)., p. 271
In one study in patients with cirrhosis or extrahepatic biliary obstruction, the t1/2alpha of nafcillin averaged 0.26 or 0.29 hr, respectively, and the t1/2beta averaged 1.2 and 1.7 hr, respectively. Serum clearance of the drug in these patients was lower than in patients with normal renal and hepatic function and averaged 291.5 ml/min in those with cirrhosis and 163.4 ml/min in those with extrahepatic obstruction.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 95. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1995 (Plus Supplements 1995)., p. 271
In children 1 mo to 14 yr of age, the serum half-life of nafcillin ranges from 0.75-1.9 hr. Serum concn of nafcillin are generally higher and the serum half-life is longer in neonates than in older children. In one study, the serum half-life of nafcillin ranged from 2.2-5.5 hr in neonates 3 wk of age or younger and 1.2-2.3 hr in neonates 4-9 wk of age.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 95. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1995 (Plus Supplements 1995)., p. 271
Like other penicillins, nafcillin exerts a bactericidal action against penicillin-susceptible microorganisms during the state of active multiplication in the bacterial cell wall synthesis. It inhibits the biosynthesis of the bacterial cell wall by forming covalent bonds with penicillin-binding proteins that play a critical role in the final transpeptidation process. Binding to penicillin-binding proteins inhibits the transpeptidase and carboxypeptidase activities conferred by these proteins and prevents the formation of the crosslinks.
SODIUM NAFCILLIN & METHICILLIN ARE GOOD EXAMPLES OF A STRUCTURAL DESIGN THAT INHIBITS BETA-LACTAM HYDROLYSIS BY STERIC CROWDING. /SODIUM NAFCILLIN/
Casarett, L.J., and J. Doull. Toxicology: The Basic Science of Poisons. New York: MacMillan Publishing Co., 1975., p. 115
The penicillins and their metabolites are potent immunogens because of their ability to combine with proteins and act as haptens for acute antibody-mediated reactions. The most frequent (about 95 percent) or "major" determinant of penicillin allergy is the penicilloyl determinant produced by opening the beta-lactam ring of the penicillin. This allows linkage of the penicillin to protein at the amide group. "Minor" determinants (less frequent) are the other metabolites formed, including native penicillin and penicilloic acids. /Penicillins/
Haddad, L.M., Clinical Management of Poisoning and Drug Overdose. 2nd ed. Philadelphia, PA: W.B. Saunders Co., 1990., p. 953
Bactericidal; inhibit bacterial cell wall synthesis. Action is dependent on the ability of penicillins to reach and bind penicillin-binding proteins (PBPs) located on the inner membrane of the bacterial cell wall. Penicillin-binding proteins (which include transpeptidases, carboxypeptidases, and endopeptidases) are enzymes that are involved in the terminal stages of assembling the bacterial cell wall and in reshaping the cell wall during growth and division. Penicillins bind to, and inactivate, penicillin-binding proteins, resulting in the weakening of the bacterial cell wall and lysis. /Penicillins/
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 2150