
1. Hydrochloride, Nafoxidine
2. Nafoxidine Hydrochloride
3. U 11,100a
4. U 11000a
5. U-11,100a
6. U-11000a
7. U11,100a
8. U11000a
1. 1845-11-0
2. Nafoxidine [inn]
3. Nafoxidinum [inn-latin]
4. Nafoxidina [inn-spanish]
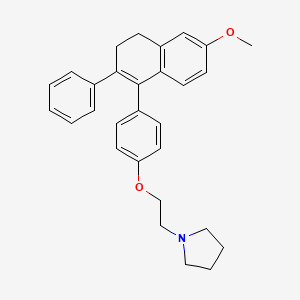
5. 1-[2-[4-(6-methoxy-2-phenyl-3,4-dihydronaphthalen-1-yl)phenoxy]ethyl]pyrrolidine
6. U-11000a
7. Pyrrolidine, 1-(2-(4-(3,4-dihydro-6-methoxy-2-phenyl-1-naphthalenyl)phenoxy)ethyl)-
8. Chembl28211
9. 4riy10wm82
10. Chebi:34881
11. Nsc 70735
12. 1-(2-(p-(3,4-dihydro-6-methoxy-2-phenyl-1-naphthyl)phenoxy)ethyl)pyrrolidine
13. 1-(2-(4-(6-methoxy-2-phenyl-3,4-dihydronaphthalen-1-yl)phenoxy)ethyl)pyrrolidine
14. 1-{2-[4-(6-methoxy-2-phenyl-3,4-dihydronaphthalen-1-yl)phenoxy]ethyl}pyrrolidine
15. Pyrrolidine, 1-(2-(p-(3,4-dihydro-6-methoxy-2-phenyl-1-naphthyl)phenoxy)ethyl)-
16. Pyrrolidine,1-[2-[4-(3,4-dihydro-6-methoxy-2-phenyl-1-naphthalenyl)phenoxy]ethyl]-
17. Nafoxidina
18. Nafoxidinum
19. Nsc70735
20. Brn 1440873
21. Unii-4riy10wm82
22. Spectrum5_000222
23. Nafoxidine [who-dd]
24. 5-20-01-00210 (beilstein Handbook Reference)
25. Bidd:er0063
26. Schembl153125
27. 11100a (*hydrochloride*)
28. Gtpl4263
29. Dtxsid7022386
30. U 11100 (*hydrochloride*)
31. U 11100a (*hydrochloride*)
32. U-11100a (*hydrochloride*)
33. Zinc538045
34. Bdbm50065941
35. Ncgc00188970-01
36. 845n110
37. Q6958201
38. 1-(2-(4-(3,4-dihydro-6-methoxy-2-phenyl-1-naphthalenyl)phenoxy)ethyl)pyrrolidine
39. 1-(2-[4-(6-methoxy-2-phenyl-3,4-dihydro-1-naphthalenyl)phenoxy]ethyl)pyrrolidine #
40. 1-{2-[4-(6-methoxy-2-phenyl-3,4-dihydro-naphthalen-1-yl)-phenoxy]-ethyl}-pyrrolidine
| Molecular Weight | 425.6 g/mol |
|---|---|
| Molecular Formula | C29H31NO2 |
| XLogP3 | 5.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 7 |
| Exact Mass | 425.235479232 g/mol |
| Monoisotopic Mass | 425.235479232 g/mol |
| Topological Polar Surface Area | 21.7 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 611 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antineoplastic Agents, Hormonal
Antineoplastic agents that are used to treat hormone-sensitive tumors. Hormone-sensitive tumors may be hormone-dependent, hormone-responsive, or both. A hormone-dependent tumor regresses on removal of the hormonal stimulus, by surgery or pharmacological block. Hormone-responsive tumors may regress when pharmacologic amounts of hormones are administered regardless of whether previous signs of hormone sensitivity were observed. The major hormone-responsive cancers include carcinomas of the breast, prostate, and endometrium; lymphomas; and certain leukemias. (From AMA Drug Evaluations Annual 1994, p2079) (See all compounds classified as Antineoplastic Agents, Hormonal.)
Estrogen Antagonists
Compounds which inhibit or antagonize the action or biosynthesis of estrogenic compounds. (See all compounds classified as Estrogen Antagonists.)
