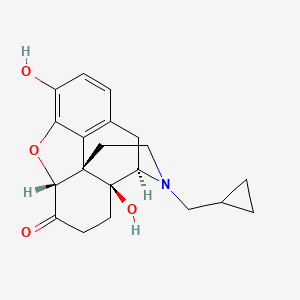

1. Antaxone
2. Celupan
3. En 1639a
4. En-1639a
5. En1639a
6. Nalorex
7. Naltrexone Hydrochloride
8. Nemexin
9. Revia
10. Trexan
1. 16590-41-3
2. Vivitrol
3. Revia
4. N-cyclopropylmethylnoroxymorphone
5. Vivitrex
6. Celupan
7. N-cyclopropylmethyl-14-hydroxydihydromorphinone
8. 17-(cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxymorphinan-6-one
9. 17-(cyclopropylmethyl)-4,5alpha-epoxy-3,14-dihydroxymorphinan-6-one
10. 5s6w795cqm
11. Chebi:7465
12. En-1639a Free Base
13. Nemexin
14. Nsc-758439
15. (4r,4as,7ar,12bs)-3-(cyclopropylmethyl)-4a,9-dihydroxy-2,4,5,6,7a,13-hexahydro-1h-4,12-methanobenzofuro[3,2-e]isoquinolin-7-one
16. Um-792
17. En-1639a [as Hydrochloride]
18. Dsstox_cid_26313
19. Dsstox_rid_81532
20. Dsstox_gsid_46313
21. Naltrexonum [inn-latin]
22. (4r,4as,7ar)-3-(cyclopropylmethyl)-4a,9-dihydroxy-2,4,5,6,7a,13-hexahydro-1h-4,12-methanobenzofuro[3,2-e]isoquinolin-7-one
23. Naltrexona [inn-spanish]
24. Naltrexona
25. Naltrexonum
26. (-)-naltrexone
27. Morphinan-6-one, 17-(cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxy-, (5.alpha.)-
28. (1s,5r,13r,17s)-4-(cyclopropylmethyl)-10,17-dihydroxy-12-oxa-4-azapentacyclo[9.6.1.0^{1,13}.0^{5,17}.0^{7,18}]octadeca-7(18),8,10-trien-14-one
29. Naltrexone [usan:inn:ban]
30. Naltrexone Base Anhydrous
31. Cas-16590-41-3
32. Ccris 3506
33. Hsdb 6750
34. Einecs 240-649-9
35. Brn 3596648
36. Unii-5s6w795cqm
37. (-)naltrexone
38. Pti-555
39. Ncgc00162274-02
40. Vivitrol (tn)
41. Naltrexone [mi]
42. Naltrexone [inn]
43. (4r,4as,7ar,12bs)-3-(cyclopropylmethyl)-4a,9-dihydroxy-2,4,5,6,7a,13-hexahydro-1h-4,12-methanobenzofuro[3,2-e]isoquinoline-7-one;hydrochloride
44. Naltrexone (usan/inn)
45. Prestwick0_000116
46. Prestwick1_000116
47. Prestwick2_000116
48. Prestwick3_000116
49. Naltrexone [hsdb]
50. Naltrexone [usan]
51. Naltrexone [vandf]
52. Naltrexone [mart.]
53. Naltrexone [usp-rs]
54. Naltrexone [who-dd]
55. Us9107954, Naltrexone
56. Schembl34681
57. Bspbio_000132
58. Bidd:gt0405
59. Chembl19019
60. Spbio_002071
61. Bpbio1_000146
62. Gtpl1639
63. Zinc1773
64. 17-(cyclopropylmethyl)-4,5.alpha.-epoxy-3,14-dihydroxymorphinan-6-one
65. Dtxsid4046313
66. Naltrexone [orange Book]
67. Bdbm60212
68. Cid_5485201
69. Cyclopropylmethyl(dihydroxy)[?]one
70. Hms2089o11
71. Bcp07022
72. Ex-a4863
73. Tox21_112007
74. Bdbm50000787
75. Mfcd00242996
76. Pdsp2_000847
77. Akos015994596
78. Morphinan-6-one, 17-(cyclopropylmethyl)-4,5-alpha-epoxy-3,14-dihydroxy-
79. Tox21_112007_1
80. Cs-0880
81. Db00704
82. Hs-0002
83. Naltrexone Component Of Contrave
84. Nsc 758439
85. Morphinan-6-one, 17-(cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxy-, (5alpha)-
86. Smp1_000206
87. Ncgc00024427-03
88. Ncgc00024427-04
89. Ncgc00024427-05
90. Ac-36726
91. Hy-76711
92. C07253
93. D05113
94. Ab00174152-14
95. Ab00174152_17
96. Ar-270/43507956
97. Q409587
98. Brd-k88172511-003-21-1
99. Brd-k88172511-310-03-8
100. 3,14-dihydroxy-17-(cyclopropylmethyl)-4,5alpha-epoxymorphinan-6-one
101. 17-(cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxmorphinan-6-one, (5.alpha.)-
102. Naltrexone Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
103. (4r,4as,7ar,12bs)-3-(cyclopropylmethyl)-4a,9-bis(oxidanyl)-2,4,5,6,7a,13-hexahydro-1h-4,12-methanobenzofuro[3,2-e]isoquinoline-7-one;hydrochloride
104. (4r,4as,7ar,12bs)-3-(cyclopropylmethyl)-4a,9-dihydroxy-2,3,4,4a,5,6-hexahydro-1h-4,12-methanobenzofuro[3,2-e]isoquinolin-7(7ah)-one
105. (4r,4as,7ar,12bs)-3-(cyclopropylmethyl)-4a,9-dihydroxy-2,4,5,6,7a,13-hexahydro-1h-4,12-methanobenzofuro[3,2-e]isoquinoline-7-one
106. 4-(cyclopropylmethyl)-10,17-dihydroxy-12-oxa-4-azapentacyclo[9.6.1.0~1,13~.0~5,17~.0~7,18~]octadeca-7(18),8,10-trien-14-one
| Molecular Weight | 341.4 g/mol |
|---|---|
| Molecular Formula | C20H23NO4 |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Exact Mass | 341.16270821 g/mol |
| Monoisotopic Mass | 341.16270821 g/mol |
| Topological Polar Surface Area | 70 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 621 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Revia |
| PubMed Health | Naltrexone |
| Drug Classes | Ethanol Dependency, Opioid Dependency, Toxicology-Antidote Agent |
| Drug Label | REVIA (naltrexone hydrochloride tablets USP), an opioid antagonist, is a synthetic congener of oxymorphone with no opioid agonist properties. Naltrexone differs in structure from oxymorphone in that the methyl group on the nitrogen atom is replaced... |
| Active Ingredient | Naltrexone hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Teva Womens |
| 2 of 4 | |
|---|---|
| Drug Name | Vivitrol |
| PubMed Health | Naltrexone |
| Drug Classes | Ethanol Dependency, Opioid Dependency, Toxicology-Antidote Agent |
| Active Ingredient | Naltrexone |
| Dosage Form | For suspension, extended release |
| Route | Intramuscular |
| Strength | 380mg/vial |
| Market Status | Prescription |
| Company | Alkermes |
| 3 of 4 | |
|---|---|
| Drug Name | Revia |
| PubMed Health | Naltrexone |
| Drug Classes | Ethanol Dependency, Opioid Dependency, Toxicology-Antidote Agent |
| Drug Label | REVIA (naltrexone hydrochloride tablets USP), an opioid antagonist, is a synthetic congener of oxymorphone with no opioid agonist properties. Naltrexone differs in structure from oxymorphone in that the methyl group on the nitrogen atom is replaced... |
| Active Ingredient | Naltrexone hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Teva Womens |
| 4 of 4 | |
|---|---|
| Drug Name | Vivitrol |
| PubMed Health | Naltrexone |
| Drug Classes | Ethanol Dependency, Opioid Dependency, Toxicology-Antidote Agent |
| Active Ingredient | Naltrexone |
| Dosage Form | For suspension, extended release |
| Route | Intramuscular |
| Strength | 380mg/vial |
| Market Status | Prescription |
| Company | Alkermes |
Narcotic Antagonists
National Library of Medicine's Medical Subject Headings. Naltrexone. Online file (MeSH, 2017). Available from, as of Oct 4, 2017: https://meshb.nlm.nih.gov/search
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Naltrexone is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of August 30, 2017: https://clinicaltrials.gov/
Naltrexone hydrochloride is designated an orphan drug by the US Food and Drug Administration (FDA) and is used orally for its opiate antagonist effects as an adjunct to a medically supervised behavior modification program in the maintenance of opiate cessation (opiate-free state) in individuals formerly physically dependent on opiates and who have successfully undergone detoxification. /Included in US product label/
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2318
Naltrexone is used orally or im in the management of alcohol dependence in conjunction with a comprehensive management program that includes psychosocial support. /Included in US product label/
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2318
For more Therapeutic Uses (Complete) data for Naltrexone (31 total), please visit the HSDB record page.
Naltrexone hydrochloride is contraindicated in: 1. Patients receiving opioid analgesics. 2. Patients currently dependent on opioids, including those currently maintained on opiate agonists (e.g., methadone ) or partial agonists (e.g., buprenorphine). 3. Patients in acute opioid withdrawal. 4. Any individual who has failed the naloxone challenge test or who has a positive urine screen for opioids. 5. Any individual with a history of sensitivity to naltrexone hydrochloride or any other components of this product. It is not known if there is any cross-sensitivity with naloxone or the phenanthrene containing opioids.
NIH; DailyMed. Current Medication Information for Naltrexone hydrochloride tablet, film coated (Updated: February 2017). Available from, as of October 12, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=49aa3d6d-2270-4615-aafa-b440859ab870
Cases of hepatitis and clinically significant liver dysfunction were observed in association with naltrexone hydrochloride exposure during the clinical development program and in the postmarketing period. Transient, asymptomatic hepatic transaminase elevations were also observed in the clinical trials and postmarketing period. When patients presented with elevated transaminases, there were often other potential causative or contributory etiologies identified, including pre-existing alcoholic liver disease, hepatitis B and/or C infection, and concomitant usage of other potentially hepatotoxic drugs. Although clinically significant liver dysfunction is not typically recognized as a manifestation of opioid withdrawal, opioid withdrawal that is precipitated abruptly may lead to systemic sequelae, including acute liver injury. Patients should be warned of the risk of hepatic injury and advised to seek medical attention if they experience symptoms of acute hepatitis. Use of naltrexone hydrochloride should be discontinued in the event of symptoms and/or signs of acute hepatitis.
NIH; DailyMed. Current Medication Information for Naltrexone hydrochloride tablet, film coated (Updated: February 2017). Available from, as of October 12, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=49aa3d6d-2270-4615-aafa-b440859ab870
An increase in naltrexone AUC of approximately 5- and 10-fold in patients with compensated and decompensated liver cirrhosis, respectively, compared with subjects with normal liver function has been reported. These data also suggest that alterations in naltrexone bioavailability are related to liver disease severity.
NIH; DailyMed. Current Medication Information for Naltrexone hydrochloride tablet, film coated (Updated: February 2017). Available from, as of October 12, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=49aa3d6d-2270-4615-aafa-b440859ab870
Studies to evaluate possible interactions between naltrexone hydrochloride and drugs other than opiates have not been performed. Consequently, caution is advised if the concomitant administration of naltrexone hydrochloride and other drugs is required. The safety and efficacy of concomitant use of naltrexone hydrochloride and disulfiram is unknown, and the concomitant use of two potentially hepatotoxic medications is not ordinarily recommended unless the probable benefits outweigh the known risks. Lethargy and somnolence have been reported following doses of naltrexone hydrochloride and thioridazine. Patients taking naltrexone hydrochloride may not benefit from opioid containing medicines, such as cough and cold preparations, antidiarrheal preparations, and opioid analgesics. In an emergency situation when opioid analgesia must be administered to a patient receiving naltrexone hydrochloride, the amount of opioid required may be greater than usual, and the resulting respiratory depression may be deeper and more prolonged.
NIH; DailyMed. Current Medication Information for Naltrexone hydrochloride tablet, film coated (Updated: February 2017). Available from, as of October 12, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=49aa3d6d-2270-4615-aafa-b440859ab870
For more Drug Warnings (Complete) data for Naltrexone (36 total), please visit the HSDB record page.
Used as an adjunct to a medically supervised behaviour modification program in the maintenance of opiate cessation in individuals who were formerly physically dependent on opiates and who have successfully undergone detoxification. Also used for the management of alcohol dependence in conjunction with a behavioural modification program.
FDA Label
Naltrexone, a pure opioid antagonist, is a synthetic congener of oxymorphone with no opioid agonist properties. Naltrexone is indicated in the treatment of alcohol dependence and for the blockade of the effects of exogenously administered opioids. It markedly attenuates or completely blocks, reversibly, the subjective effects of intravenously administered opioids. When co-administered with morphine, on a chronic basis, naltrexone blocks the physical dependence to morphine, heroin and other opioids. In subjects physically dependent on opioids, naltrexone will precipitate withdrawal symptomatology.
Alcohol Deterrents
Substances interfering with the metabolism of ethyl alcohol, causing unpleasant side effects thought to discourage the drinking of alcoholic beverages. Alcohol deterrents are used in the treatment of alcoholism. (See all compounds classified as Alcohol Deterrents.)
Narcotic Antagonists
Agents inhibiting the effect of narcotics on the central nervous system. (See all compounds classified as Narcotic Antagonists.)
N - Nervous system
N07 - Other nervous system drugs
N07B - Drugs used in addictive disorders
N07BB - Drugs used in alcohol dependence
N07BB04 - Naltrexone
Absorption
Although well absorbed orally, naltrexone is subject to significant first pass metabolism with oral bioavailability estimates ranging from 5 to 40%.
Route of Elimination
Both parent drug and metabolites are excreted primarily by the kidney (53% to 79% of the dose), however, urinary excretion of unchanged naltrexone accounts for less than 2% of an oral dose and fecal excretion is a minor elimination pathway. The renal clearance for naltrexone ranges from 30 to 127 mL/min and suggests that renal elimination is primarily by glomerular filtration.
Volume of Distribution
1350 L [intravenous administration]
Clearance
~ 3.5 L/min [after IV administration]
Naltrexone hydrochloride is rapidly and almost completely (about 96%) absorbed from the GI tract following oral administration, but the drug undergoes extensive first-pass metabolism in the liver. Only 5-40% of an orally administered dose reaches systemic circulation unchanged. Considerable interindividual variation in absorption of the drug during the first 24 hours after a single dose has been reported. The bioavailability of naltrexone hydrochloride tablets is reportedly similar to that of an oral solution of the drug (not commercially available in the US).
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2325
Peak plasma concentrations of naltrexone and 6-beta-naltrexol (the major metabolite of naltrexone) usually occur within 1 hour following oral administration of the tablets and 0.6 hours following oral administration of the solution. Because orally administered naltrexone undergoes substantial first-pass metabolism, plasma concentrations of 6-beta-naltrexol following oral administration are substantially higher than corresponding concentrations of naltrexone. Following oral administration, the area under the serum concentration-time curve (AUC) for 6-beta-naltrexol is 10-30 times greater than the AUC for naltrexone. Following single- or multiple-dose (i.e., once daily) oral administration of naltrexone hydrochloride 50 mg in healthy individuals, peak plasma concentrations of naltrexone and 6-beta-naltrexol averaged 10.6-13.7 and 109-139 ng/mL, respectively.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2325
Little, if any, accumulation of naltrexone and/or 6-beta-naltrexol appears to occur following chronic administration of the drug. Following chronic administration of naltrexone, plasma concentrations of 6-beta-naltrexol are at least 40% higher than those following administration of a single dose of the drug; however, plasma concentrations of naltrexone and 6-beta-naltrexol 24 hours after each dose of chronically administered drug are similar to concentrations 24 hours after a single dose of the drug in most patients.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2325
Naltrexone hydrochloride is widely distributed throughout the body, but considerable interindividual variation in distribution parameters during the first 24 hours following a single oral dose has been reported. Following subcutaneous administration of radiolabeled drug in rats, the drug distributes into CSF within 30 minutes. In animals, CSF naltrexone concentrations are reported to be approximately 30% of concurrent peak plasma concentrations. The drug and its metabolites have been shown to distribute into saliva and erythrocytes following oral administration in humans.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2325
For more Absorption, Distribution and Excretion (Complete) data for Naltrexone (13 total), please visit the HSDB record page.
Hepatic. When administered orally, naltrexone undergoes extensive biotransformation and is metabolized to 6 beta-naltrexol (which may contribute to the therapeutic effect) and other minor metabolites.
Naltrexone is metabolized in the liver principally by reduction of the 6-keto group of naltrexone to 6-beta-naltrexol (6-beta-hydroxynaltrexone). Naltrexone also undergoes metabolism by catechol-O-methyl transferase (COMT) to form 2-hydroxy-3-methoxy-6-beta-naltrexol (HMN) and 2-hydroxy-3-methoxynaltrexone. Several minor metabolites have also been identified, including noroxymorphone and 3-methoxy-6-beta-naltrexol. Because oral but not im administration of naltrexone results in substantial first-pass hepatic metabolism of the drug, 6-beta-naltrexol concentrations following im administration are substantially lower than concentrations of the metabolite obtained following oral administration. Naltrexone does not appear to inhibit or induce its own metabolism following chronic administration. Cytochrome P-450 (CYP) isoenzymes are not involved in the metabolism of naltrexone. Naltrexone and its metabolites undergo conjugation with glucuronic acid. The major fraction of total drug and metabolites in both plasma and urine consists of conjugated metabolites. The drug and its metabolites may undergo enterohepatic circulation.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2325
Metabolites of naltrexone may contribute to the opiate antagonist activity of the drug. Like naltrexone, 6-beta-naltrexol is an essentially pure opiate antagonist, with a potency of 6-8% that of naltrexone in precipitating withdrawal symptoms in dogs physically dependent on morphine and 1.25-2% that of naltrexone in mice. Because of its weak affinity for opiate receptors, 2-hydroxy-3-methoxy-6-beta-naltrexol (HMN) may not contribute appreciably to the opiate antagonist activity of naltrexone; however, the in vivo opiate antagonist activity of HMN or 2-hydroxy-3-methoxynaltrexone has not been studied. Noroxymorphone, a minor metabolite of naltrexone, is a potent opiate agonist and may be responsible for the agonist activity (eg, miosis) that occurs infrequently in individuals receiving naltrexone.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2325
Naltrexone and its metabolites (unconjugated and conjugated) are excreted principally in urine via glomerular filtration; 6-beta-naltrexol, conjugated 6-beta-naltrexol, and conjugated naltrexone are also excreted via tubular secretion. Naltrexone may also undergo partial reabsorption by the renal tubules. Following single- or multiple-dose oral administration of naltrexone hydrochloride, respectively, approximately 38-60 or 70% of a dose has been recovered in urine, principally as 6-beta-naltrexol (conjugated and unconjugated). Most urinary excretion of naltrexone occurs within the first 4 hours after oral administration. Less than 2% of an orally administered dose is excreted unchanged in urine within 24 hours. Approximately 5-10, 19-35, 7-16, 3.5-4.6, and 0.45% of an oral dose are excreted in urine as conjugated naltrexone, 6-beta-naltrexol, conjugated 6-beta-naltrexol, 2-hydroxy-3-methoxy-6-beta-naltrexol (HMN), and 2-hydroxy-3-methoxynaltrexone, respectively, within 24 hours. Less than 5% of a dose is excreted in feces, principally as 6-beta-naltrexol, within 24 hours following single- or multiple-dose oral administration of the drug. Following oral administration of 50 mg of radiolabeled naltrexone in one patient, approximately 93% of the radiolabeled dose was excreted within 133 hours; about 79 and 14% were excreted in urine and feces, respectively.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2325-6
Following im administration of naltrexone extended-release injection, the half-life of naltrexone and 6-beta-naltrexol is 5-10 days.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2326
For more Metabolism/Metabolites (Complete) data for Naltrexone (6 total), please visit the HSDB record page.
Naltrexone has known human metabolites that include Naltrexone-3-glucuronide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
4 hours for naltrexone and 13 hours for the active metabolite 6 beta-naltrexol.
Plasma concentrations of naltrexone and 6-beta-naltrexol, the major metabolite, appear to decline in a biphasic manner during the first 24 hours following a single oral dose or during chronic administration of the drug. Following oral administration of single or multiple doses of naltrexone hydrochloride, the plasma half-lives of naltrexone and 6-beta-naltrexol in the initial phase (t1/2 alpha) average 1.1-3.9 and 2.3-3.1 hours, respectively, and the plasma half-lives in the terminal phase (t1/2 beta) average 9.7-10.3 and 11.4-16.8 hours, respectively. Plasma concentrations of naltrexone and 6-beta-naltrexol have also been reported to decline in a triphasic manner following oral administration, with a terminal elimination half-life after the first 24 hours of 96 hours for naltrexone and 18 hours for 6-beta-naltrexol, possibly resulting from initial distribution into body tissues and subsequent redistribution into systemic circulation.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2325
Pharmacokinetics of naltrexone hydrochloride (NTX) and naltrexone glucuronide was studied in the dog using HPLC-electrochemical detection with naloxone as internal standard. After iv 5 mg or po 10 mg NTX, ... the elimination half-lives of NTX were 78 +/- 6 min and 74 +/- 6 min, respectively. ... The major metabolite of NTX in dog plasma was beta-glucuronidase-hydrolyzable conjugate. Dosing NTX intravenously and orally, ... the elimination half-lives of the glucuronide from plasma were 3.4 hr and 12.6 hr, respectively.
PMID:9208648 Li H et al; Yao Xue Xue Bao 31 (4): 254-7 (1996)
Naltrexone is a pure opiate antagonist and has little or no agonist activity. The mechanism of action of naltrexone in alcoholism is not understood; however, involvement of the endogenous opioid system is suggested by preclinical data. Naltrexone is thought to act as a competitive antagonist at mc, , and receptors in the CNS, with the highest affintiy for the receptor. Naltrexone competitively binds to such receptors and may block the effects of endogenous opioids. This leads to the antagonization of most of the subjective and objective effects of opiates, including respiratory depression, miosis, euphoria, and drug craving. The major metabolite of naltrexone, 6--naltrexol, is also an opiate antagonist and may contribute to the antagonistic activity of the drug.
Naltrexone competes for opiate receptors and displaces opioid drugs from these receptors, thus reversing their effects. It is capable of antagonizing all opiate receptors.
Papich, M.G. Saunders Handbook of Veterinary Drugs Small and Large Animal. 3rd ed. St. Louis, MO: Elsevier Saunders, 2011, p. 533