


1. Antaxone
2. Celupan
3. En 1639a
4. En-1639a
5. En1639a
6. Nalorex
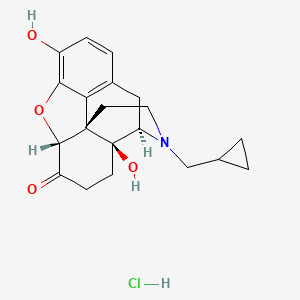
7. Naltrexone
8. Nemexin
9. Revia
10. Trexan
1. Naltrexone Hcl
2. 16676-29-2
3. Trexan
4. Depade
5. Antaxone
6. Revia
7. Naltrexone (hydrochloride)
8. En-1639a
9. Nih 8503
10. Celupan
11. Nemexin
12. N-cyclopropylmethyl-noroxymorphone Hydrochloride
13. Mls000069607
14. Naltrexone Hydrochloride [usp]
15. Z6375yw9sf
16. Vivitrex
17. 17-(cyclopropylmethyl)-4,5-alpha-epoxy-3,14-dihydroxy-morphinan-6-one Hydrochloride
18. Nalorex
19. Smr000058767
20. En-1639a (as Hydrochloride)
21. En 1639a
22. (4r,4as,7ar,12bs)-3-(cyclopropylmethyl)-4a,9-dihydroxy-2,4,5,6,7a,13-hexahydro-1h-4,12-methanobenzofuro[3,2-e]isoquinolin-7-one;hydrochloride
23. Naltrexone Hydrochloride (usp)
24. (4r,4as,7ar,12bs)-3-(cyclopropylmethyl)-4a,9-dihydroxy-2,3,4,4a,5,6-hexahydro-1h-4,12-methanobenzofuro[3,2-e]isoquinolin-7(7ah)-one Hydrochloride
25. Morphinan-6-one, 17-(cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxy-, Hydrochloride (1:1), (5alpha)-
26. Ccris 1168
27. Einecs 240-723-0
28. Unii-z6375yw9sf
29. Naltrel
30. Naltrexone.hcl
31. Naltrexone Depot
32. Prestwick_348
33. Mfcd00069324
34. (5alpha)-17-(cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxymorphinan-6-one Hydrochloride
35. Revia (tn)
36. Xr-ntx
37. (4r,4as,7ar,12bs)-3-(cyclopropylmethyl)-4a,9-dihydroxy-2,4,5,6,7a,13-hexahydro-1h-4,12-methanobenzofuro[3,2-e]isoquinoline-7-one;hydrochloride
38. Opera_id_1828
39. Naltrexone Monohydrochloride
40. Morphinan-6-one, 17-(cyclopropylmethyl)-4,5-alpha-epoxy-3,14-dihydroxy-, Hydrochloride
41. Morphinan-6-one, 17-(cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxy-, Hydrochloride, (5-alpha)-
42. Schembl37713
43. Mls001076516
44. Mls002153483
45. Mls002695940
46. Cyto-205
47. Chembl1201149
48. Dtxsid50937236
49. Pti-901
50. Chebi:134687
51. Bcp08343
52. Naltrexone Hydrochloride [mi]
53. S2103
54. Vp-004
55. Akos015994597
56. Ccg-268383
57. Cs-0763
58. Hs-0003
59. Nc00693
60. 17-cyclopropylmethyl)-4,5alpha-epoxy-3,14-dihydroxymorphinan-6-one Hydrochloride
61. Morphinan-6-one, 17-(cyclopropylmethyl)-4,5-alpha-oxy-3,14-dihydoxy-, Hydrochloride
62. Morphinan-6-one, 17-(cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxy-, Hydrochloride, (5alpha)-
63. Naltrexone Hydrochloride [mart.]
64. Naltrexone Hydrochloride [vandf]
65. Naltrexone Hydrochloride [who-dd]
66. Bn164635
67. Hy-76710
68. Morphinan-6-one, 17-(cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxy-, Hydrochloride, (5.alpha.)-
69. N1176
70. Naltrexone Hydrochloride [green Book]
71. Sw196619-3
72. Naltrexone Hydrochloride [orange Book]
73. D02095
74. Embeda Component Naltrexone Hydrochloride
75. H10489
76. Naltrexone Hydrochloride [ep Monograph]
77. Naltrexone Hydrochloride [usp Monograph]
78. Troxyca Component Naltrexone Hydrochloride
79. 676n292
80. Contrave Component Naltrexone Hydrochloride
81. Naltrexone Hydrochloride Component Of Embeda
82. Naltrexone Hydrochloride Component Of Troxyca
83. Naltrexone Hydrochloride Component Of Contrave
84. Q27096434
85. Z1558290144
86. Naltrexone Hydrochloride 1.0 Mg/ml In Methanol (as Free Base)
87. Naltrexone Hydrochloride, European Pharmacopoeia (ep) Reference Standard
88. (5a)-17-(cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxy-morphinan-6-one Hydrochloride
89. 17-(cyclopropylmethyl)-3,14-dihydroxy-4,5alpha-epoxymorphinan-6-one Hydrochloride
90. 17-cyclopropylmethyl)-4,5.alpha.-epoxy-3,14-dihydroxymorphinan-6-one Hydrochloride
91. (1s,5r,13r,17s)-4-(cyclopropylmethyl)-10,17-dihydroxy-12-oxa-4-azapentacyclo[9.6.1.0^{1,13}.0^{5,17}.0^{7,18}]octadeca-7(18),8,10-trien-14-one Hydrochloride
92. (4r,4as,7ar,12bs)-3-(cyclopropylmethyl)-4a,9-dihydroxy-2,4,5,6,7a,13-hexahydro-1h-4,12-methanobenzofuro[3,2-e]isoquinolin-7-one;hydron;chloride
93. (5alpha,17r)-17-(cyclopropylmethyl)-3,14-dihydroxy-6-oxo-4,5-epoxymorphinan-17-ium Chloride
94. Morphinan-6-one, 17-(cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxy-, (5.alpha.)-, Hydrochloride (1:1)
| Molecular Weight | 377.9 g/mol |
|---|---|
| Molecular Formula | C20H24ClNO4 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Exact Mass | 377.1393859 g/mol |
| Monoisotopic Mass | 377.1393859 g/mol |
| Topological Polar Surface Area | 70 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 621 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 6 | |
|---|---|
| Drug Name | Naltrexone hydrochloride |
| PubMed Health | Naltrexone |
| Drug Classes | Ethanol Dependency, Opioid Dependency, Toxicology-Antidote Agent |
| Drug Label | Naltrexone hydrochloride, an opioid antagonist, is a synthetic congener of oxymorphone with no opioid agonist properties. Naltrexone differs in structure from oxymorphone in that the methyl group on the nitrogen atom is replaced by a cyclopropylmethy... |
| Active Ingredient | Naltrexone hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Accord Hlthcare; Mallinckrodt; Sun Pharma Global; Elite Labs; Barr |
| 2 of 6 | |
|---|---|
| Drug Name | Revia |
| PubMed Health | Naltrexone |
| Drug Classes | Ethanol Dependency, Opioid Dependency, Toxicology-Antidote Agent |
| Drug Label | REVIA (naltrexone hydrochloride tablets USP), an opioid antagonist, is a synthetic congener of oxymorphone with no opioid agonist properties. Naltrexone differs in structure from oxymorphone in that the methyl group on the nitrogen atom is replaced... |
| Active Ingredient | Naltrexone hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Teva Womens |
| 3 of 6 | |
|---|---|
| Drug Name | Vivitrol |
| Active Ingredient | Naltrexone |
| Dosage Form | For suspension, extended release |
| Route | Intramuscular |
| Strength | 380mg/vial |
| Market Status | Prescription |
| Company | Alkermes |
| 4 of 6 | |
|---|---|
| Drug Name | Naltrexone hydrochloride |
| PubMed Health | Naltrexone |
| Drug Classes | Ethanol Dependency, Opioid Dependency, Toxicology-Antidote Agent |
| Drug Label | Naltrexone hydrochloride, an opioid antagonist, is a synthetic congener of oxymorphone with no opioid agonist properties. Naltrexone differs in structure from oxymorphone in that the methyl group on the nitrogen atom is replaced by a cyclopropylmethy... |
| Active Ingredient | Naltrexone hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Accord Hlthcare; Mallinckrodt; Sun Pharma Global; Elite Labs; Barr |
| 5 of 6 | |
|---|---|
| Drug Name | Revia |
| PubMed Health | Naltrexone |
| Drug Classes | Ethanol Dependency, Opioid Dependency, Toxicology-Antidote Agent |
| Drug Label | REVIA (naltrexone hydrochloride tablets USP), an opioid antagonist, is a synthetic congener of oxymorphone with no opioid agonist properties. Naltrexone differs in structure from oxymorphone in that the methyl group on the nitrogen atom is replaced... |
| Active Ingredient | Naltrexone hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Teva Womens |
| 6 of 6 | |
|---|---|
| Drug Name | Vivitrol |
| Active Ingredient | Naltrexone |
| Dosage Form | For suspension, extended release |
| Route | Intramuscular |
| Strength | 380mg/vial |
| Market Status | Prescription |
| Company | Alkermes |
Alcohol Deterrents
Substances interfering with the metabolism of ethyl alcohol, causing unpleasant side effects thought to discourage the drinking of alcoholic beverages. Alcohol deterrents are used in the treatment of alcoholism. (See all compounds classified as Alcohol Deterrents.)
Narcotic Antagonists
Agents inhibiting the effect of narcotics on the central nervous system. (See all compounds classified as Narcotic Antagonists.)
