
1. Aleve
2. Anaprox
3. Methoxypropiocin
4. Naprosin
5. Naprosyn
6. Naproxen Sodium
7. Naproxenate, Sodium
8. Proxen
9. Sodium Naproxenate
10. Sodium, Naproxen
11. Synflex
1. 22204-53-1
2. (s)-naproxen
3. Naprosyn
4. (+)-naproxen
5. Equiproxen
6. Naproxene
7. (s)-2-(6-methoxynaphthalen-2-yl)propanoic Acid
8. Aleve
9. (s)-(+)-naproxen
10. (s)-(+)-2-(6-methoxy-2-naphthyl)propionic Acid
11. Laraflex
12. D-naproxen
13. Ec-naprosyn
14. Naproxenum
15. Calosen
16. Naprosyne
17. Naproxeno
18. Nycopren
19. Bonyl
20. Naixan
21. Reuxen
22. (2s)-2-(6-methoxynaphthalen-2-yl)propanoic Acid
23. Axer
24. (+)-(s)-naproxen
25. Naproxen(+)
26. Acusprain
27. Anexopen
28. Arthrisil
29. Artrixen
30. Artroxen
31. Bipronyl
32. Clinosyn
33. Danaprox
34. Flexipen
35. Leniartil
36. Novonaprox
37. Anaprox
38. Apronax
39. Artagen
40. Atiflan
41. Congex
42. Daprox
43. Genoxen
44. Lefaine
45. Nafasol
46. Nalyxan
47. Napflam
48. Napmel
49. Naposin
50. Napren
51. Naprius
52. Veradol
53. Fuxen
54. Naxen
55. Naxyn
56. Xenar
57. Dysmenalgit N
58. Apo-naproxen
59. Floginax
60. Rs-3540
61. (s)-2-(6-methoxy-2-naphthyl)propionic Acid
62. (+)-2-(6-methoxy-2-naphthyl)propionic Acid
63. (+)-2-(methoxy-2-naphthyl)-propionic Acid
64. (s)-6-methoxy-alpha-methyl-2-naphthaleneacetic Acid
65. Dysmenalgit
66. Diocodal
67. (s)-2-(6-methoxy-2-naphthyl)propanoic Acid
68. Prexan
69. Apo-napro-na
70. D-2-(6-methoxy-2-naphthyl)propionic Acid
71. (+)-2-(methoxy-2-naphthyl)-propionsaeure
72. (+)-(s)-6-methoxy-alpha-methyl-2-naphthaleneacetic Acid
73. (s)-(+)-6-methoxy-alpha-methyl-2-naphthaleneacetic Acid
74. Proxen
75. Cg 3117
76. Chebi:7476
77. (2s)-2-(6-methoxy-2-naphthyl)propanoic Acid
78. Chembl154
79. Nsc-750183
80. Nsc-757239
81. 57y76r9atq
82. D-2-(6'-methoxy-2'-naphthyl)-propionsaeure
83. Pn400 Component Naproxen
84. Naprium
85. Naprux
86. Propionic Acid, 2-(6-methoxy-2-naphthyl)-, (+)-
87. Duk
88. 2-naphthaleneacetic Acid, 6-methoxy-.alpha.-methyl-, (s)-
89. Headlon
90. Napratec
91. Naprontag
92. Narocin
93. Naxopren
94. Prafena
95. Priaxen
96. Pronaxen
97. Rheumaflex
98. Saritilron
99. Sinartrin
100. Soproxen
101. Sutolin
102. Tohexen
103. Traumox
104. Flexen
105. Napxen
106. Noflam
107. Patxen
108. Rahsen
109. Sinton
110. Sutony
111. Velsay
112. Vinsen
113. Narma
114. Roxen
115. Anax
116. 2-naphthaleneacetic Acid, 6-methoxy-.alpha.-methyl-, (.alpha.s)-
117. Cas-22204-53-1
118. Flanax Forte
119. Napren E
120. Naprosyn Lle
121. Proxen Le
122. Naxen F
123. Proxen Lle
124. U-ritis
125. Naprosyn Lle Forte
126. (s)-2-(6-methoxy-naphthalen-2-yl)-propionic Acid
127. Naproxi 250
128. Naproxi 500
129. (s)-6-methoxy-.alpha.-methyl-2-naphthaleneacetic Acid
130. Naxyn 250
131. Naxyn 500
132. Equiproxen (veterinary)
133. Naproxene [inn-french]
134. Naproxenum [inn-latin]
135. (+)-6-methoxy-alpha-methyl-2-naphthaleneacetic Acid
136. Naproxeno [inn-spanish]
137. 26159-31-9
138. Xenar-cr
139. Ccris 5265
140. Hsdb 3369
141. Einecs 244-838-7
142. Rs 3540
143. (s)-(+)-6-methoxy-alp.-methyl-2-naphthalene Acetic Acid
144. Unii-57y76r9atq
145. Naprolag
146. Naprosine
147. Naprosy
148. Panoxen
149. Proxine
150. Mnpa
151. Sr-01000075977
152. Dl Naproxen
153. Naprosyn (tn)
154. Naproxen,(s)
155. Nps
156. Prestwick_349
157. (+)-2-(methoxy-2-naphthyl)-propionsaeure [german]
158. Mfcd00010500
159. Naproxen [usan:usp:inn:ban:jan]
160. D-2-(6'-methoxy-2'-naphthyl)-propionsaeure [german]
161. 2-naphthaleneacetic Acid, 6-methoxy-alpha-methyl-, (+)-
162. 2-naphthaleneacetic Acid, 6-methoxy-alpha-methyl-, (s)-
163. Spectrum_000977
164. 4or0
165. 4po0
166. Naproxen [hsdb]
167. Naproxen [inci]
168. Naproxen [usan]
169. (s)-(+)-naproxene
170. Naproxen [inn]
171. Naproxen [jan]
172. Naproxen [mi]
173. Naproxen [vandf]
174. Prestwick0_000791
175. Prestwick1_000791
176. Prestwick2_000791
177. Spectrum2_001043
178. Spectrum3_000514
179. Spectrum4_000069
180. Spectrum5_001327
181. (+)naproxen
182. Naproxen [mart.]
183. Naproxen2-(6-methoxy-naphthalen-2-yl)-propionic Acid
184. Epitope Id:139974
185. Naproxen [usp-rs]
186. Naproxen [who-dd]
187. Upcmld-dp001
188. 2-(6-methoxy-naphthalen-2-yl)-propionic Acid(naproxen)
189. Ec 244-838-7
190. Schembl3046
191. Dsstox_cid_20686
192. Dsstox_rid_79542
193. Dsstox_gsid_40686
194. Lopac0_000792
195. Bspbio_002067
196. Kbiogr_000597
197. Kbioss_001457
198. Bidd:gt0062
199. Divk1c_000242
200. Naproxen [green Book]
201. Spectrum1500425
202. Naproxen (jp17/usp/inn)
203. Spbio_000966
204. Spbio_002861
205. Naproxen [orange Book]
206. Gtpl5230
207. Naproxen [ep Monograph]
208. Vimovo Component Naproxen
209. Dtxsid4040686
210. Naproxen [usp Monograph]
211. Upcmld-dp001:001
212. Cmwtzpsulfxxja-vifpvbqesa-
213. Hms500m04
214. Kbio1_000242
215. Kbio2_001457
216. Kbio2_004025
217. Kbio2_006593
218. Kbio3_001567
219. Naproxen 1.0 Mg/ml In Methanol
220. Ninds_000242
221. Hms1920p13
222. Hms2089n21
223. Hms2091h12
224. Hms3649m13
225. Hms3886c15
226. Pharmakon1600-01500425
227. Zinc105216
228. Act02593
229. Naproxen Component Of Vimovo
230. Tox21_301953
231. Bdbm50339185
232. Ccg-40130
233. Nsc750183
234. Nsc757239
235. Akos005267223
236. Ac-1363
237. Db00788
238. Me-0100
239. Nsc 750183
240. Nsc 757239
241. Sdccgsbi-0050769.p005
242. Idi1_000242
243. Naproxen 100 Microg/ml In Acetonitrile
244. Naproxen Solution 1.0 Mg/ml In Methanol
245. Ncgc00016759-01
246. Ncgc00016759-02
247. Ncgc00016759-03
248. Ncgc00016759-38
249. Ncgc00021127-01
250. Ncgc00161591-01
251. Ncgc00255562-01
252. Hy-15030
253. Naproxen, Meets Usp Testing Specifications
254. Sbi-0050769.p004
255. Am20060551
256. M1021
257. S5177
258. Naproxen, Vetranal(tm), Analytical Standard
259. (2s)-2-(6-methoxynaphth-2-yl)propanoic Acid
260. (s)-2-(6-methoxy-2-naphthyl)-propionic Acid
261. (s)-2-(6-methoxynaphthalen-2-yl)propanoicacid
262. 04n531
263. C01517
264. D00118
265. O10203
266. (2s)-2-(6-methoxy(2-naphthyl))propanoic Acid
267. Ab00052049-04
268. Ab00052049_05
269. (+)(s)2-(6-methoxy-2-naphthyl)-propionic Acid
270. 2-(6-methoxy-2-naphthyl)propanoic Acid , (+)-
271. Sr-01000003110
272. Q-201447
273. Q1215575
274. Sr-01000003110-5
275. Sr-01000075977-3
276. Sr-01000075977-4
277. (+)-6-methoxy-.alpha.-methyl-2-napthaleneacetic Acid
278. Brd-k59197931-001-02-9
279. Brd-k59197931-001-03-7
280. Brd-k59197931-236-09-6
281. Sr-01000075977-10
282. (+)-6-methoxy-.alpha.-methyl-2-naphthaleneacetic Acid
283. (alphas)-6-methoxy-alpha-methyl-2-naphthaleneacetic Acid
284. (s)-(+)-2-(6-methoxy-naphthalen-2-yl)-propionic Acid
285. (s)-6-methoxy-.alpha.-methyl-2-naphthalene Acetic Acid
286. Naproxen, British Pharmacopoeia (bp) Reference Standard
287. Naproxen, European Pharmacopoeia (ep) Reference Standard
288. (s)-(+)-6-methoxy-alpha-methyl-2-naphthaleneacetic Acid, 98%
289. 2-naphthaleneacetic Acid, 6-methoxy-.alpha.-methyl-, (+)-
290. Naproxen, United States Pharmacopeia (usp) Reference Standard
291. (+)-(s)-6-methoxy-.alpha.-methyl-2-naphthaleneacetic Acid
292. Naproxen, Pharmaceutical Secondary Standard; Certified Reference Material
293. Naproxen Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
294. (s)-(+)-2-(6-methoxy-2-naphthyl)propionic Acid;(s)-(+)-6-methoxy-alpha-methyl-2-naphthaleneacetic Acid
295. 131991-52-1
296. D-naproxen; (2s)-2-(6-methoxynaphthalen-2-yl)propanoic Acid; (s)-6-methoxy-?-methyl-2-naphthaleneacetic Acid; D-2-(6-methoxy-2-naphthyl)propionic Acid
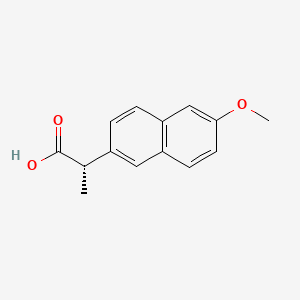
| Molecular Weight | 230.26 g/mol |
|---|---|
| Molecular Formula | C14H14O3 |
| XLogP3 | 3.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 230.094294304 g/mol |
| Monoisotopic Mass | 230.094294304 g/mol |
| Topological Polar Surface Area | 46.5 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 277 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 10 | |
|---|---|
| Drug Name | Aleve |
| PubMed Health | Naproxen (By mouth) |
| Drug Classes | Analgesic, Antimigraine, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent |
| Active Ingredient | Naproxen sodium |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 200mg base |
| Market Status | Over the Counter |
| Company | Bayer |
| 2 of 10 | |
|---|---|
| Drug Name | Ec-naprosyn |
| Drug Label | Naproxen is a proprionic acid derivative related to the arylacetic acid group of nonsteroidal anti-inflammatory drugs.The chemical names for naproxen and naproxen sodium are (S)-6-methoxy--methyl-2-naphthaleneacetic acid and (S)-6-methoxy--methyl... |
| Active Ingredient | Naproxen |
| Dosage Form | Tablet, delayed release |
| Route | Oral |
| Strength | 375mg; 500mg |
| Market Status | Prescription |
| Company | Roche Palo |
| 3 of 10 | |
|---|---|
| Drug Name | Naprelan |
| Drug Label | NAPRELAN* Tablets contain naproxen sodium, a member of the arylacetic acid group of nonsteroidal anti-inflammatory drugs (NSAIDs). NAPRELAN Tablets use the proprietary IPDAS** (Intestinal Protective Drug Absorption System) technology. It is a r... |
| Active Ingredient | Naproxen sodium |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | eq 750mg base; eq 500mg base; eq 375mg base |
| Market Status | Prescription |
| Company | Almatica Pharma |
| 4 of 10 | |
|---|---|
| Drug Name | Naprosyn |
| Drug Label | Naproxen is a proprionic acid derivative related to the arylacetic acid group of nonsteroidal anti-inflammatory drugs.The chemical names for naproxen and naproxen sodium are (S)-6-methoxy--methyl-2-naphthaleneacetic acid and (S)-6-methoxy--methyl... |
| Active Ingredient | Naproxen |
| Dosage Form | Tablet; Suspension |
| Route | Oral |
| Strength | 250mg; 375mg; 500mg; 25mg/ml |
| Market Status | Prescription |
| Company | Roche Palo |
| 5 of 10 | |
|---|---|
| Drug Name | Naproxen |
| Drug Label | NAPRELAN* Tablets contain naproxen sodium, a member of the arylacetic acid group of nonsteroidal anti-inflammatory drugs (NSAIDs). NAPRELAN Tablets use the proprietary IPDAS** (Intestinal Protective Drug Absorption System) technology. It is a r... |
| Active Ingredient | Naproxen |
| Dosage Form | Tablet; Suspension; Tablet, delayed release |
| Route | Oral |
| Strength | 250mg; 375mg; 500mg; 25mg/ml |
| Market Status | Prescription |
| Company | Mylan Pharms; Marksans Pharma; Teva; Sandoz; Invagen Pharms; Hikma Pharms; Roxane; Perrigo R And D; Glenmark Generics; Amneal Pharms Ny; Aurobindo Pharma Usa; Zydus Pharms Usa; Pliva; Mylan |
| 6 of 10 | |
|---|---|
| Drug Name | Aleve |
| PubMed Health | Naproxen (By mouth) |
| Drug Classes | Analgesic, Antimigraine, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent |
| Active Ingredient | Naproxen sodium |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 200mg base |
| Market Status | Over the Counter |
| Company | Bayer |
| 7 of 10 | |
|---|---|
| Drug Name | Ec-naprosyn |
| Drug Label | Naproxen is a proprionic acid derivative related to the arylacetic acid group of nonsteroidal anti-inflammatory drugs.The chemical names for naproxen and naproxen sodium are (S)-6-methoxy--methyl-2-naphthaleneacetic acid and (S)-6-methoxy--methyl... |
| Active Ingredient | Naproxen |
| Dosage Form | Tablet, delayed release |
| Route | Oral |
| Strength | 375mg; 500mg |
| Market Status | Prescription |
| Company | Roche Palo |
| 8 of 10 | |
|---|---|
| Drug Name | Naprelan |
| Drug Label | NAPRELAN* Tablets contain naproxen sodium, a member of the arylacetic acid group of nonsteroidal anti-inflammatory drugs (NSAIDs). NAPRELAN Tablets use the proprietary IPDAS** (Intestinal Protective Drug Absorption System) technology. It is a r... |
| Active Ingredient | Naproxen sodium |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | eq 750mg base; eq 500mg base; eq 375mg base |
| Market Status | Prescription |
| Company | Almatica Pharma |
| 9 of 10 | |
|---|---|
| Drug Name | Naprosyn |
| Drug Label | Naproxen is a proprionic acid derivative related to the arylacetic acid group of nonsteroidal anti-inflammatory drugs.The chemical names for naproxen and naproxen sodium are (S)-6-methoxy--methyl-2-naphthaleneacetic acid and (S)-6-methoxy--methyl... |
| Active Ingredient | Naproxen |
| Dosage Form | Tablet; Suspension |
| Route | Oral |
| Strength | 250mg; 375mg; 500mg; 25mg/ml |
| Market Status | Prescription |
| Company | Roche Palo |
| 10 of 10 | |
|---|---|
| Drug Name | Naproxen |
| Drug Label | NAPRELAN* Tablets contain naproxen sodium, a member of the arylacetic acid group of nonsteroidal anti-inflammatory drugs (NSAIDs). NAPRELAN Tablets use the proprietary IPDAS** (Intestinal Protective Drug Absorption System) technology. It is a r... |
| Active Ingredient | Naproxen |
| Dosage Form | Tablet; Suspension; Tablet, delayed release |
| Route | Oral |
| Strength | 250mg; 375mg; 500mg; 25mg/ml |
| Market Status | Prescription |
| Company | Mylan Pharms; Marksans Pharma; Teva; Sandoz; Invagen Pharms; Hikma Pharms; Roxane; Perrigo R And D; Glenmark Generics; Amneal Pharms Ny; Aurobindo Pharma Usa; Zydus Pharms Usa; Pliva; Mylan |
Anti-Inflammatory Agents, Non-Steroidal; Cyclooxygenase Inhibitors; Gout Suppressants
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Naproxen and its salt are used to relieve postoperative pain (including that associated with dental surgery), postpartum pain, primary dysmenorrhea, pain following insertion of an intrauterine contraceptive device, orthopedic pain, headache (including migraine), and visceral pain associated with cancer. Naproxen sodium also may be used for self-medication to provide temporary relief of minor aches and pains associated with the common cold, headache, toothache, muscular aches, and backache. /Included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2164
Naproxen has been used in the symptomatic management of osteitis deformans (Paget's disease of bone) and Bartter's syndrome. /Use is not currently included in the labeling approved by the US FDA/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2164
When used in the treatment of rheumatoid arthritis or juvenile rheumatoid arthritis, naproxen has relieved pain and stiffness, reduced swelling, and improved mobility and grip strength. In the treatment of osteoarthritis, naproxen has relieved pain and stiffness and improved knee joint function. Naproxen appears to be only palliative in these conditions and has not been shown to permanently arrest or reverse the underlying disease process. Naproxen sodium also may be used for self-medication to provide temporary relief of minor aches and pains associated with arthritis. /Included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2164
For more Therapeutic Uses (Complete) data for NAPROXEN (9 total), please visit the HSDB record page.
Pseudoporphyria is characterized by skin fragility, blistering and scarring in sun-exposed skin areas without abnormalities in porphyrin metabolism. The phenylpropionic acid derivative group of nonsteroidal anti-inflammatory drugs, especially naproxen, is known to cause pseudoporphyria. Naproxen is currently one of the most prescribed drugs in the therapy of juvenile idiopathic arthritis. The prevalence of pseudoporphyria was determined in a 9-year retrospective study of children with juvenile idiopathic arthritis and associated diseases. In addition, /the investigators/ prospectively studied the incidence of pseudoporphyria in 196 patients (127 girls and 69 boys) with juvenile idiopathic arthritis and associated diseases treated with naproxen from July 2001 to March 2002. ... These data /were compared/ with those from a matched control group with juvenile idiopathic arthritis and associated diseases not treated with naproxen in order to identify risk factors for development of pseudoporphyria. The incidence of pseudoporphyria in the group of children taking naproxen was 11.4%. Pseudoporphyria was particularly frequent in children with the early-onset pauciarticular subtype of juvenile idiopathic arthritis (mean age 4.5 years). Pseudoporphyria was associated with signs of disease activity, such as reduced hemoglobin (<11.75 g/dL), and increased leucocyte counts (>10,400/uL) and erythocyte sedimentation rate (>26 mm/hour). Comedications, especially chloroquine intake, appeared to be additional risk factors. The mean duration of naproxen therapy before the onset of pseudoporphyria was 18.1 months, and most children with pseudoporphyria developed their lesions within the first 2 years of naproxen treatment. Juvenile idiopathic arthritis disease activity seems to be a confounding factor for pseudoporphyria. In particular, patients with early-onset pauciarticular juvenile idiopathic arthritis patients who have significant inflammation appear to be prone to developing pseudoporphyria upon treatment with naproxen.
PMID:17266758 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1860069 Schad SG et al; Arthritis Res Ther 9 (1): R10 (2007).
Short-term use of NSAIAs to relieve acute pain, especially at low dosages, does not appear to be associated with an increased risk of serious cardiovascular events (except immediately following coronary artery bypass graft (CABG) surgery). Therefore, in early 2005, the US Food and Drug Administration (FDA) concluded that preparations of NSAIAs (including naproxen) that currently were available without a prescription had a favorable benefit-to-risk ratio when used according to labeled instructions, and determined that these preparations should remain available without a prescription despite the addition of a boxed warning to the professional labeling of prescription-only preparations of these drugs.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2165
With the exception of precautions related to the sodium content of naproxen sodium, the cautions associated with naproxen sodium use are the same as those for naproxen use. Each 275 or 550 mg naproxen sodium tablet contains about 1 or 2 mEq of sodium, respectively, and each mL of the commercially available naproxen suspension contains about 0.34 mEq of sodium; this should be considered in patients whose sodium intake must be restricted. /Naproxen sodium/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2166
Patients should be advised that naproxen, like other nonsteroidal anti-inflammatory agents, is not free of potential adverse effects, including some that can cause discomfort, and that, rarely, more serious effects (e.g., GI bleeding), which may require hospitalization and may even be fatal, can occur. Patients also should be informed that, while nonsteroidal anti-inflammatory agents may be commonly employed for conditions that are less serious, nonsteroidal anti-inflammatory agent therapy often is considered essential for the management of some diseases (eg, rheumatoid arthritis), and the drugs have a major role in the management of pain. Clinicians may wish to discuss with their patients the potential risks and likely benefits of nonsteroidal anti-inflammatory agent therapy, particularly when consideration is being given to use of these drugs in less serious conditions for which therapy without a nonsteroidal anti-inflammatory agent may represent an acceptable alternative to both the patient and clinician.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2166-7
For more Drug Warnings (Complete) data for NAPROXEN (40 total), please visit the HSDB record page.
Naproxen is indicated for the management of rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, polyarticular juvenile idiopathic arthritis, tendinitis, bursitis, acute gout, primary dysmenorrhea, and for the relief of mild to moderate pain. Further, it is first-line therapy for osteoarthritis, acute gouty arthritis, dysmenorrhea, and musculoskeletal inflammation and pain.
Naproxen is an established non-selective NSAID and is useful as an analgesic, anti-inflammatory and antipyretic. Similar to other NSAIDs, the pharmacological activity of naproxen can be attributed to the inhibition of cyclo-oxygenase, which in turn reduces prostaglandin synthesis in various tissues and fluids including the synovial fluid, gastric mucosa, and the blood. Although naproxen is an effective analgesic, it can have unintended deleterious effects in the patient. For instance, naproxen can adversely affect blood pressure control. A study found that use of naproxen induced an increase in blood pressure, although the increase was not as significant as that found with ibuprofen use. Further, studies have found that the risk of upper gastrointestinal bleeding is on average four-fold higher for individuals taking NSAIDs. Other factors that increase the risk of upper gastrointestinal bleeding include concurrent use of corticosteroids or anticoagulants, and a history of gastrointestinal ulcers.
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Cyclooxygenase Inhibitors
Compounds or agents that combine with cyclooxygenase (PROSTAGLANDIN-ENDOPEROXIDE SYNTHASES) and thereby prevent its substrate-enzyme combination with arachidonic acid and the formation of eicosanoids, prostaglandins, and thromboxanes. (See all compounds classified as Cyclooxygenase Inhibitors.)
Gout Suppressants
Agents that increase uric acid excretion by the kidney (URICOSURIC AGENTS), decrease uric acid production (antihyperuricemics), or alleviate the pain and inflammation of acute attacks of gout. (See all compounds classified as Gout Suppressants.)
M01AE02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
G - Genito urinary system and sex hormones
G02 - Other gynecologicals
G02C - Other gynecologicals
G02CC - Antiinflammatory products for vaginal administration
G02CC02 - Naproxen
M - Musculo-skeletal system
M01 - Antiinflammatory and antirheumatic products
M01A - Antiinflammatory and antirheumatic products, non-steroids
M01AE - Propionic acid derivatives
M01AE02 - Naproxen
M - Musculo-skeletal system
M02 - Topical products for joint and muscular pain
M02A - Topical products for joint and muscular pain
M02AA - Antiinflammatory preparations, non-steroids for topical use
M02AA12 - Naproxen
Absorption
Naproxen is available as a free acid and sodium salt. At comparable doses, (naproxen 500 mg = naproxen sodium 550 mg) they differ slightly in their rates of absorption, but otherwise they are therapeutically and pharmacologically equivalent. Naproxen sodium achieves a peak plasma concentration after 1 hour, while peak plasma concentration is observed after 2 hours with naproxen (free acid). There are no differences between the 2 forms in the post-absorption phase pharmacokinetics. The difference in initial absorption should be considered when treating acute pain, since naproxen sodium may offer a quicker onset of action. The mean Cmax for the various formulations (immediate release, enteric coated, controlled release etc.) of naproxen are comparable and range from 94 mcg/mL to 97.4 mcg/mL. In one pharmacokinetic study, the mean Tmax of naproxen 500 mg (immediate release) given every 12 hours over 5 days was 3 hours, compared to a mean Tmax of 5 hours for Naprelan 1000 mg (controlled release) given every 24 hours over 5 days. In this same study, the AUC0-24hr was 1446mcgxhr/mL for naproxen immediate release and 1448 mcgxhr/mL for the controlled release formulation. A separate study comparing the pharmacokinetics of Naprosyn tablets and EC-Naprosyn observed the following values: Tmax and AUC0-12hrs of EC-Naprosyn were 4 hours and 845 mcgxhr/mL respectively, and Tmax and AUC0-12hrs values of Naprosyn were 1.9 hours and 767 mcgxhr/mL respectively. When given in combination with sumatriptan the Cmax of naproxen is roughly 36% lower compared to naproxen sodium 550 mg tablets, and the median Tmax is 5 hours. Based on the AUC and Cmax of naproxen, Vimovo (naproxen/esomeprazole combination product) and enteric-coated naproxen may be considered bioequivalent. Overall, naproxen is rapidly and completely absorbed when administered orally and rectally. Food may contribute to a delay in the absorption of orally administered naproxen, but will not affect the extent of absorption.
Route of Elimination
After oral administration, about 95% of naproxen and it's metabolites can be recovered in the urine with 66-92% recovered as conjugated metabolite and less than 1% recovered as naproxen or desmethylnaproxen. Less than 5% of naproxen is excreted in the feces.
Volume of Distribution
Naproxen has a volume of distribution of 0.16 L/kg.
Clearance
Naproxen is cleared at a rate of 0.13 mL/min/kg.
Oral absorption of naproxen in dogs is rapid, with peak plasma concentration reached in 0.5-3 hr. The reported elimination half-life in dogs is 34-72 hr. Naproxen is highly protein bound (>99.0%). In dogs, naproxen is primarily eliminated through the bile, whereas in other species, the primary route of elimination is through the kidneys. The long half-life of naproxen in dogs appears to be due to its extensive enterohepatic recirculation.
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2528
After therapeutic doses, naproxen is more than 99% bound to plasma proteins. When naproxen binding sites become saturated (at twice daily doses of 500 mg or more), plasma free drug concentrations increase and may result in increased urinary clearance rates. Therefore, plasma naproxen concentrations tend to plateau when dosage exceeds 500 mg twice daily. In a study in patients with severe renal failure, binding of naproxen to serum proteins was decreased compared to healthy adults; the decreased binding may have accounted for an increase in metabolism and apparent volume of distribution of the drug observed in these patients. In patients with chronic alcoholic liver disease, total plasma concentrations of naproxen are decreased while concentrations of the unbound drug are increased.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2170
The pharmacokinetics of naproxen, its metabolite 6-hydroxy-alpha-methyl-2-naphthaleneacetic acid (O-desmethylnaproxen), and their acyl glucuronides were studied in 10 subjects (ages 20-50 yr) who received an oral dose of 500 mg naproxen. Mean half-life of naproxen in 9 subjects was 24.7 hr. A half-life of 7.4 hr in the 10th subject was considered an extraordinary case. Naproxen acyl glucuronide accounted for 50.8% of the dose, its isomerized conjugate isoglucuronide for 6.5%, O-desmethylnaproxen acyl glucuronide for 14.3%, and its isoglucuronide for 5.5%. Excretion of naproxen and O-desmethylnaproxen was negligible. Plasma protein binding was 98% for naproxen, 100% for O-desmethylnaproxen, 92% for naproxen acyl glucuronide, 66% for naproxen isoglucuronide, 72% for O-desmethylnaproxen acyl glucuronide, and 42% for O-desmethylnaproxen isoglucuronide. It was concluded that naproxen is O-desmethylated and parent drug and metabolite are conjugated into acyl glucuronides.
PMID:8218967 Vree TB et al; Biopharm Drug Dispos 14 (8): 491-502 (1993)
The effects of rheumatoid arthritis disease activity on the pharmacokinetics of the highly albumin-bound nonsteroidal anti-inflammatory drug naproxen were studied in six patients during chronic therapy. In the same patients, kinetics during active disease were compared with those in improvement. Active disease is commonly associated with hypoalbuminemia: 30 +/- 4 gm/L vs. 41 +/- 2 gm/L (mean +/- SD) at the time of improvement. Total naproxen concentrations were significantly lower in active disease, together with a larger apparent volume of distribution (10.6 +/- 1.8 L vs. 8.4 +/- 1.3 L; P less than 0.05) and total body clearance (0.79 +/- 1.8 L/hr vs. 0.59 +/- 0.14 L/hr; P less than 0.001). Peak unbound naproxen concentrations were 29% +/- 19% (P less than 0.05) lower at the time of improvement. The unbound clearance was found diminished during active disease (390 +/- 277 L/hr) in comparison with improvement (488 +/- 343 L/hr; P less than 0.05). Clinical implications of the alterations in naproxen kinetics induced by polyarticular inflammation in patients with rheumatoid arthritis are discussed.
PMID:3335121 Van den Ouweland FA et al; Clin Pharmacol Ther 43 (1): 79-85 (1988).
For more Absorption, Distribution and Excretion (Complete) data for NAPROXEN (15 total), please visit the HSDB record page.
Naproxen is heavily metabolized in the liver and undergoes both Phase I and Phase II metabolism. The first step involves demethylation of naproxen via CYP 1A2, 2C8, and 2C9. Both naproxen and desmethylnaproxen proceed to Phase II metabolism; however, desmethylnaproxen can form both acyl and phenolic glucoronide products, while naproxen only produces the acyl glucuronide. The acyl glucuronidation process involves UGT 1A1, 1A3, 1A6, 1A7, 1A9, 1A10 and 2B7, while phenolic glucuronidation is catalyzed by UGT 1A1, 1A7,1A9, and 1A10. Desmethylnaproxen also undergoes sulphation which is mediated by SULT 1A1, 1B1 and 1E1.
Naproxen is extensively metabolized in the liver to 6-desmethylnaproxen. Approximately 95% of the drug is excreted in urine as unchanged naproxen (less than 1%) and 6-desmethylnaproxen (less than 1%) and their glucuronide or other conjugates (66-92%). Some data suggest that renal excretion of unchanged naproxen may be negligible or absent; previously reported concentrations of unchanged drug may reflect rapid hydrolysis of conjugates during collection, storage, and handling of urine samples. The half-life of naproxen metabolites and conjugates is shorter than 12 hours. Naproxen metabolites may accumulate in patients with renal impairment. Elimination of naproxen is reduced in patients with severe renal impairment. A small amount (less than 5%) of the drug is excreted in feces.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2170
The pharmacokinetics of naproxen, its metabolite 6-hydroxy-alpha-methyl-2-naphthaleneacetic acid (O-desmethylnaproxen), and their acyl glucuronides were studied in 10 subjects (ages 20-50 yr) who received an oral dose of 500 mg naproxen. Mean half-life of naproxen in 9 subjects was 24.7 h. A half-life of 7.4 h in the 10th subject was considered an extraordinary case. Naproxen acyl glucuronide accounted for 50.8% of the dose, its isomerized conjugate isoglucuronide for 6.5%, O-desmethylnaproxen acyl glucuronide for 14.3%, and its isoglucuronide for 5.5%. Excretion of naproxen and O-desmethylnaproxen was negligible. Plasma protein binding was 98% for naproxen, 100% for O-desmethylnaproxen, 92% for naproxen acyl glucuronide, 66% for naproxen isoglucuronide, 72% for O-desmethylnaproxen acyl glucuronide, and 42% for O-desmethylnaproxen isoglucuronide. It was concluded that naproxen is O-desmethylated and parent drug and metabolite are conjugated into acyl glucuronides.
PMID:8218967 Vree TB et al; Biopharm Drug Dispos 14 (8): 491-502 (1993)
Naproxen has known human metabolites that include (2S,3S,4S,5R)-3,4,5-Trihydroxy-6-[(2S)-2-(6-methoxynaphthalen-2-yl)propanoyl]oxyoxane-2-carboxylic acid and O-Desmethylnaproxen.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The elimination half-life of naproxen is reported to be 12-17 hours.
The reported elimination half-life in dogs is 34-72 hr.
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2528
In healthy adults, the plasma half-life of naproxen reportedly ranges from 10-20 hr. The manufacturer state that the plasma half-life of naproxen is about 13 hr. The plasma half-life and elimination of the drug appear to be similar in children and adults.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2170
The pharmacokinetics of naproxen, its metabolite 6-hydroxy-alpha-methyl-2-naphthaleneacetic acid (O-desmethylnaproxen), and their acyl glucuronides were studied in 10 subjects (ages 20-50 yr) who received an oral dose of 500 mg naproxen. Mean half-life of naproxen in 9 subjects was 24.7 hr. A half-life of 7.4 hr in the 10th subject was considered an extraordinary case. ...
PMID:8218967 Vree TB et al; Biopharm Drug Dispos 14 (8): 491-502 (1993)
As with other non-selective NSAIDs, naproxen exerts it's clinical effects by blocking COX-1 and COX-2 enzymes leading to decreased prostaglandin synthesis. Although both enzymes contribute to prostaglandin production, they have unique functional differences. The COX-1 enzymes is constitutively active and can be found in normal tissues such as the stomach lining, while the COX-2 enzyme is inducible and produces prostaglandins that mediate pain, fever and inflammation. The COX-2 enzyme mediates the desired antipyretic, analgesic and anti-inflammatory properties offered by Naproxen, while undesired adverse effects such as gastrointestinal upset and renal toxicities are linked to the COX-1 enzyme.
Naproxen has pharmacologic actions similar to those of other prototypical nonsteroidal anti-inflammatory agents (NSAIAs). The drug exhibits anti-inflammatory, analgesic, and antipyretic activity. The exact mechanisms have not been clearly established, but many of the actions appear to be associated principally with the inhibition of prostaglandin synthesis. Naproxen inhibits the synthesis of prostaglandins in body tissues by inhibiting cyclooxygenase; at least 2 isoenzymes, cyclooxygenase-1 (COX-1) and -2 (COX-2) (also referred to as prostaglandin G/H synthase-1 (PGHS-1) and -2 (PGHS-2), respectively), have been identified that catalyze the formation of prostaglandins in the arachidonic acid pathway. Naproxen, like other prototypical NSAIAs, inhibits both COX-1 and COX-2. Although the exact mechanisms have not been clearly established, NSAIAs appear to exert anti-inflammatory, analgesic, and antipyretic activity principally through inhibition of the COX-2 isoenzyme; COX-1 inhibition presumably is responsible for the drugs' unwanted effects on GI mucosa and platelet aggregation.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2169
The anti-inflammatory, analgesic, and antipyretic effects of naproxen and other nonsteroidal anti-inflammatory agents (NSAIAs), including selective inhibitors of COX-2 (e.g., celecoxib, rofecoxib), appear to result from inhibition of prostaglandin synthesis. While the precise mechanism of the anti-inflammatory and analgesic effects of NSAIAs continues to be investigated, these effects appear to be mediated principally through inhibition of the COX-2 isoenzyme at sites of inflammation with subsequent reduction in the synthesis of certain prostaglandins from their arachidonic acid precursors. Naproxen stabilizes lysosomal membranes and inhibits the response of neutrophils to chemotactic stimuli. The drug does not possess glucocorticoid or adrenocorticoid-stimulating properties.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2169
Naproxen lowers body temperature in patients with fever. Although the mechanism of the antipyretic effect of nonsteroidal anti-inflammatory agents is not known, it has been suggested that suppression of prostaglandin synthesis in the CNS (probably in the hypothalamus) may be involved.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2169
Naproxen-induced inhibition of prostaglandin synthesis may result in decreased frequency and intensity of uterine contractility. Prostaglandins E2 and F2alpha increase the amplitude and frequency of uterine contractions in pregnant women; current evidence suggests that primary dysmenorrhea is also mediated by these prostaglandins. Whether the increased production of prostaglandins associated with primary dysmenorrhea is mediated by COX-1 or COX-2 remains to be determined. Blood concentrations of a metabolite of prostaglandin F2alpha have been found to decrease in women with dysmenorrhea who were receiving naproxen. Therapy with naproxen has been effective in relieving menstrual pain and has reduced blood loss in women with menorrhagia, probably by inhibiting the formation of these prostaglandins. Administration of naproxen during late pregnancy may prolong gestation by inhibiting uterine contractions.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2169