


1. 4-methylsalinomycin
2. Narasin A
3. Narasin Monosodium Salt
1. Narasin A
2. Compound 79891
3. Monteban
4. 55134-13-9
5. Antibiotic A-28086 Factor A
6. Antibiotic C 7819b
7. Antibiotic A 28086a
8. Salinomycin, 4-methyl-, (4s)-
9. Lilly 79891
10. 4-methylsalinomycin
11. C-7819b
12. Dzy9vu539p
13. Narasine
14. Narasino
15. Narasinum
16. Compd-79891
17. C 7819b
18. Salinomycin, 4-methyl-, (4s)
19. Monteban (tn)
20. Narasine [inn-french]
21. Narasinum [inn-latin]
22. Narasino [inn-spanish]
23. Narasin (usp/inn)
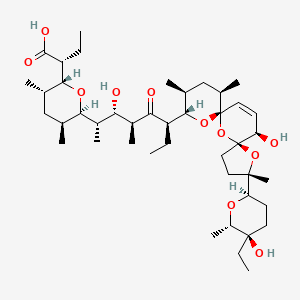
24. (2r)-2-[(2r,3s,5s,6r)-6-[(2s,3s,4s,6r)-6-[(3s,5s,7r,9s,10s,12r,15r)-3-[(2r,5r,6s)-5-ethyl-5-hydroxy-6-methyloxan-2-yl]-15-hydroxy-3,10,12-trimethyl-4,6,8-trioxadispiro[4.1.57.35]pentadec-13-en-9-yl]-3-hydroxy-4-methyl-5-oxooctan-2-yl]-3,5-dimethyloxan-2-yl]butanoic Acid
25. Unii-dzy9vu539p
26. Brn 1678311
27. Ai3-29798
28. Narasin [usan:usp:inn:ban]
29. Narasin 100 Microg/ml In Acetonitrile
30. Ncgc00167550-01
31. 2h-pyran-2-acetic Acid, Alpha-ethyl-6-(5-(2-(5-ethyltetrahydro-5-hydroxy-6-methyl-2h-pyran-2-yl)-15-hydroxy-2,10,12-trimethyl-1,6,8-trioxadispiro(4.1.5.3)pentadec-13-en-9-yl)-2-hydroxy-1,3-dimethyl-4-oxoheptyl)tetrahydro-3,5-dimethyl-
32. Narasin [usan]
33. Narasin [inn]
34. Narasin [mi]
35. Narasin [mart.]
36. Narasin [usp-rs]
37. Dsstox_cid_26707
38. Dsstox_rid_81840
39. Dsstox_gsid_46707
40. Narasin [green Book]
41. Alpha-ethyl-6-(5-(2-(5-ethyltetrahydro-5-hydroxy-6-methyl-2h-pyran-2-yl)-15-hydroxy-2,10,12-trimethyl-1,6,8-trioxadispiro(4.1.5.3)pentadec-13-en-9-yl)-2-hydroxy-1,3-dimethyl-4-oxoheptyl)tetrahydro-3,5-dimethyl-2h-pyran-2-acetic Acid
42. Schembl123401
43. Narasin [usp Impurity]
44. Chembl2104423
45. Dtxsid2046707
46. Gtpl10835
47. Chebi:183815
48. Tox21_112545
49. Zinc85540282
50. Db11432
51. Ncgc00167550-06
52. Ncgc00167550-08
53. Cas-55134-13-9
54. Hy-121410
55. Cs-0081972
56. D05122
57. Q2104314
58. (2r)-2-[(2r,3s,5s,6r)-6-[(2s,3s,4s,6r)-6-[(3s,5s,7r,9s,10s,12r,15r)-3-[(2r,5r,6s)-5-ethyl-5-hydroxy-6-methyloxan-2-yl]-15-hydroxy-3,10,12-trimethyl-4,6,8-trioxadispiro[4.1.5^{7}.3^{5}]pentadec-13-en-9
| Molecular Weight | 765.0 g/mol |
|---|---|
| Molecular Formula | C43H72O11 |
| XLogP3 | 6.2 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 12 |
| Exact Mass | 764.50746311 g/mol |
| Monoisotopic Mass | 764.50746311 g/mol |
| Topological Polar Surface Area | 161 Ų |
| Heavy Atom Count | 54 |
| Formal Charge | 0 |
| Complexity | 1360 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 19 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Coccidiostats
Agents useful in the treatment or prevention of COCCIDIOSIS in man or animals. (See all compounds classified as Coccidiostats.)