


1. 5' Nor Anhydrovinblastine
2. 5'-nor-anhydrovinblastine
3. Kw 2307
4. Kw-2307
5. Kw2307
6. Navelbine
7. Vinorelbine
8. Vinorelbine Tartrate
1. Vinorelbine Tartrate
2. 125317-39-7
3. Vinorelbine Ditartrate
4. 5'-noranhydrovinoblastine Tartrate
5. Vinorelbine Ditartrate Salt Hydrate
6. Vinorelbinetartrate
7. 1217449-57-4
8. Vinorelbine, Ditartrate
9. Vinorelbine Ditartaric Acid
10. 317v397
11. Q-100110
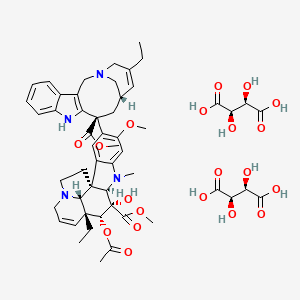
12. (2r,3r)-2,3-dihydroxybutanedioic Acid;methyl (1r,9r,10s,11r,12r,19r)-11-acetyloxy-12-ethyl-4-[(12s,14s)-16-ethyl-12-methoxycarbonyl-1,10-diazatetracyclo[12.3.1.03,11.04,9]octadeca-3(11),4,6,8,15-pentaen-12-yl]-10-hydroxy-5-methoxy-8-methyl-8,16-diazapentacyclo[10.6.1.01,9.02,7.016,19]nonadeca-2,4,6,13-tetraene-10-carboxylate
13. Methyl (3ar,3a1r,4r,5s,5ar,10br)-4-acetoxy-3a-ethyl-9-((6s,8s)-4-ethyl-8-(methoxycarbonyl)-1,3,6,7,8,9-hexahydro-2,6-methanoazecino[4,3-b]indol-8-yl)-5-hydroxy-8-methoxy-6-methyl-3a,3a1,4,5,5a,6,11,12-octahydro-1h-indolizino[8,1-cd]carbazole-5-carboxylate Bis((2r,3r)-2,3-dihydroxysuccinate)
| Molecular Weight | 1079.1 g/mol |
|---|---|
| Molecular Formula | C53H66N4O20 |
| Hydrogen Bond Donor Count | 10 |
| Hydrogen Bond Acceptor Count | 23 |
| Rotatable Bond Count | 16 |
| Exact Mass | 1078.42704051 g/mol |
| Monoisotopic Mass | 1078.42704051 g/mol |
| Topological Polar Surface Area | 364 Ų |
| Heavy Atom Count | 77 |
| Formal Charge | 0 |
| Complexity | 1820 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 12 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
| 1 of 2 | |
|---|---|
| Drug Name | VINORELBINE TARTRATE |
| Active Ingredient | VINORELBINE TARTRATE |
| Company | ACTAVIS TOTOWA (Application Number: A078011); DR REDDYS LABS LTD (Application Number: A202017); FRESENIUS KABI USA (Application Number: A076849); HOSPIRA (Application Number: A076827); JIANGSU HANSOH PHARM (Application Number: A091106); TEVA PHARMS USA (Application Number: A076028); WEST-WARD PHARMS INT (Application Number: A075992); WEST-WARD PHARMS INT (Application Number: A076461) |
| 2 of 2 | |
|---|---|
| Drug Name | NAVELBINE |
| Active Ingredient | VINORELBINE TARTRATE |
| Company | PIERRE FABRE (Application Number: N020388) |
Antineoplastic Agents, Phytogenic
Agents obtained from higher plants that have demonstrable cytostatic or antineoplastic activity. (See all compounds classified as Antineoplastic Agents, Phytogenic.)
Tubulin Modulators
Agents that interact with TUBULIN to inhibit or promote polymerization of MICROTUBULES. (See all compounds classified as Tubulin Modulators.)