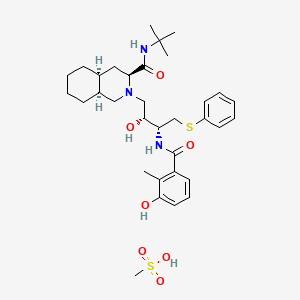

1. Ag 1343
2. Ag-1343
3. Ag1343
4. Mesylate, Nelfinavir
5. Monomethane Sulfonate, Nelfinavir
6. Nelfinavir
7. Nelfinavir Monomethane Sulfonate
8. Sulfonate, Nelfinavir Monomethane
9. Viracept
1. 159989-65-8
2. Viracept
3. Nelfinavir Mesilate
4. Nelfinavir Mesylate [usan]
5. Ag1343
6. Nelfinavir Mesylate Hydrate
7. Nelfinavir Methanesulfonate
8. Ag-1343
9. Chebi:7497
10. 98d603vp8v
11. Viracept (tn)
12. Nelfinavir Mesilate (jan)
13. Nfv
14. Dsstox_cid_10777
15. Dsstox_rid_79093
16. Dsstox_gsid_33736
17. (3s,4as,8as)-n-(tert-butyl)-2-((2r,3r)-2-hydroxy-3-(3-hydroxy-2-methylbenzamido)-4-(phenylthio)butyl)decahydroisoquinoline-3-carboxamide Methanesulfonate
18. (3s,4as,8as)-n-tert-butyl-2-((2r,3r)-3-(3,2-cresotamido)-2-hydroxy-4-(phenylthio)butyl)decahydro-3-isoquinolinecarboxamide Monomethanesulfonate (salt)
19. (3s,4as,8as)-n-tert-butyl-2-[(2r,3r)-2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-phenylsulfanylbutyl]-3,4,4a,5,6,7,8,8a-octahydro-1h-isoquinoline-3-carboxamide;methanesulfonic Acid
20. 3-isoquinolinecarboxamide, N-(1,1-dimethylethyl)decahydro-2-[(2r,3r)-2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-(phenylthio)butyl]-, (3s,4as,8as)-, Methanesulfonate (1:1)
21. Nelfinavir Mesilate [jan]
22. Nelfin
23. Chembl1205
24. Smr000469186
25. Cas-159989-65-8
26. Nsc722664
27. Hsdb 7159
28. Ncgc00090782-03
29. Unii-98d603vp8v
30. Nelfinaviri Mesilas
31. Ag 1343 Mesylate
32. Azt/3tc/nlfr Combination
33. Schembl40942
34. Mls001055355
35. Mls001401378
36. Mls006010141
37. Arv-sr0121
38. Nelfinavir Mesylate (usan/inn)
39. Hms2051n05
40. Hms2090l17
41. Hms2233m20
42. Hms3261p12
43. Hms3715b04
44. Nelfinavir Mesylate [hsdb]
45. Nelfinavir Mesylate [vandf]
46. Ex-a3335
47. Nelfinavir Mesilate [mart.]
48. Tox21_111021
49. Tox21_200921
50. Tox21_500635
51. Nelfinavir Mesilate [who-dd]
52. Nelfinavir Mesilate [who-ip]
53. S4282
54. Akos015963185
55. Tox21_111021_1
56. Ag 1341
57. Ag-1346
58. Ccg-100925
59. Ks-1089
60. Nc00175
61. Nsc-722664
62. Nelfinavir Methanesulfonate [mi]
63. Ncgc00090782-01
64. Ncgc00090782-06
65. Ncgc00258475-01
66. Ncgc00261320-01
67. Nelfinavir Mesylate [orange Book]
68. 3-isoquinolinecarboxamide, N-(1,1-dimethylethyl)decahydro-2-((2r,3r)-2-hydroxy-3-((3-hydroxy-2-methylbenzoyl)amino)-4-(phenylthio)butyl)-, (3s,4as,8as)-, Monomethanesulfonate (salt)
69. 3-isoquinolinecarboxamide, N-(1,1-dimethylethyl)decahydro-2-(2-hydroxy-3-((3-hydroxy-2-methylbenzoyl)amino)-4-(phenylthio)butyl)-, (3s-(2(2,s*,3s*),3-alpha,4a-beta,8a-beta))-, Monomethanesulfonate (salt)
70. Ac-20033
71. Ac-32581
72. Nelfinavir Mesylate (ag 1343 Mesylate)
73. Nelfinaviri Mesilas [who-ip Latin]
74. Ly-312857
75. N0986
76. Nelfinavir Mesylate Hydrate, >=98% (hplc)
77. Sw197555-2
78. C08091
79. C73028
80. D00899
81. 989n658
82. J-009662
83. Q27107510
84. (3s, 4as, 8as)-2-[(2r, 3r)-2-hydroxy-3-(3-hydroxy-2-methylbenzoylamino)-4-phenylthiobutyl]decahydroiso-quinoline-3-carboxylic Acid T-butylamide Methanesulfonate
85. (3s, 4as, 8as)-2-[(2r, 3r)-2-hydroxy-3-(3-hydroxy-2-methylbenzoylamino)-4-phenylthiobutyl]decahydroisoquinoline-3-carboxylic Acid T-butylamide Methanesulfonate
86. (3s,4as,8as)-2-[(2r,3r)-2-hydroxy-3-(3-hydroxy-2-methylbenzoylamino)-4-phenylthiobutyl]decahydroisoquinoline-3-carboxylic Acid T-butylamide Methanesulfonate
87. (3s,4as,8as)-3-(tert-butylcarbamoyl)-2-[(2r,3r)-2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-(phenylsulfanyl)butyl]decahydroisoquinolinium Methanesulfonate
88. (3s,4as,8as)-n-(1,1-dimethylethyl)decahydro-2-[(2r,3r)-2- Hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-(phenylthio)butyl]-3-isoquinolinecarboxamide Methanesulfonate
89. (3s,4as,8as)-n-tert-butyl-2-[(2r,3r)-2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-(phenylsulfanyl)butyl]decahydroisoquinoline-3-carboxamide Methanesulfonate
90. (3s,4as,8as)-n-tert-butyl-2-[(2r,3r)-2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-(phenylthio)butyl]decahydroisoquinoline-3-carboxamide Methanesulfonate (salt)
91. 3-isoquinolinecarboxamide,1-dimethylethyl)decahydro-2- [(2r,3r)-2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-(phenylthio)butyl]-, Methanesulfonate (3s,4as,8as)-
| Molecular Weight | 663.9 g/mol |
|---|---|
| Molecular Formula | C33H49N3O7S2 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 10 |
| Exact Mass | 663.30119326 g/mol |
| Monoisotopic Mass | 663.30119326 g/mol |
| Topological Polar Surface Area | 190 Ų |
| Heavy Atom Count | 45 |
| Formal Charge | 0 |
| Complexity | 922 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Viracept |
| PubMed Health | Nelfinavir (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | VIRACEPT (nelfinavir mesylate) is an inhibitor of the human immunodeficiency virus (HIV) protease.VIRACEPT Tablets are available for oral administration as a light blue, capsule-shaped tablet with a clear film coating in 250 mg strength (as nelfi... |
| Active Ingredient | Nelfinavir mesylate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 625mg base; eq 250mg base |
| Market Status | Prescription |
| Company | Agouron |
| 2 of 2 | |
|---|---|
| Drug Name | Viracept |
| PubMed Health | Nelfinavir (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | VIRACEPT (nelfinavir mesylate) is an inhibitor of the human immunodeficiency virus (HIV) protease.VIRACEPT Tablets are available for oral administration as a light blue, capsule-shaped tablet with a clear film coating in 250 mg strength (as nelfi... |
| Active Ingredient | Nelfinavir mesylate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 625mg base; eq 250mg base |
| Market Status | Prescription |
| Company | Agouron |
HIV Protease Inhibitors.
National Library of Medicine's Medical Subject Headings online file (MeSH, 2012)
HIV protease inhibitors are associated with HIV protease inhibitor-related lipodystrophy syndrome. Researchers hypothesized that liposarcomas would be similarly susceptible to the apoptotic effects of an HIV protease inhibitor, nelfinavir. We conducted a phase I trial of nelfinavir for liposarcomas. There was no limit to prior chemotherapy. The starting dose was 1,250 mg twice daily (Level 1). Doses were escalated in cohorts of three to a maximally evaluated dose of 4,250 mg (Level 5). One cycle was 28 days. Steady-state pharmacokinetics (PKs) for nelfinavir and its primary active metabolite, M8, were determined at Levels 4 (3,000 mg) and 5. Twenty subjects (13 males) were enrolled. Median (range) age was 64 years (37-81). One subject at Level 1 experienced reversible, grade 3 pancreatitis after 1 week and was replaced. No other dose-limiting toxicities were observed. Median (range) number of cycles was 3 (0.6-13.5). Overall best responses observed were 1 partial response, 1 minor response, 4 stable disease, and 13 progressive disease. Mean peak plasma levels and AUCs for nelfinavir were higher at Level 4 (7.3 mg/L; 60.9 mg/L X hr) than 5 (6.3 mg/L; 37.7 mg/L X hr). The mean ratio of M8: nelfinavir AUCs for both levels was approximately 1:3. PKs demonstrate auto-induction of nelfinavir clearance at the doses studied, although the mechanism remains unclear. Peak plasma concentrations were within range where anticancer activity was demonstrated in vitro. M8 metabolite is present at approximately 1/3 the level of nelfinavir and may also contribute to the anticancer activity observed.
PMID:22983015 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3904496 Pan J et al; Cancer Chemother Pharmacol 70 (6): 791-9 (2012)
Nelfinavir is indicated in the treatment of HIV infection when antiretroviral therapy is warranted. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1986
A phase I/II dose-ranging open-label 28-day monotherapy study of the safety, pharmacokinetics, and antiviral activity of nelfinavir mesylate (Viracept), an inhibitor of human immunodeficiency virus (HIV)-1 protease, was done in 65 HIV-1-infected subjects. After 28 days, 54 responding subjects entered an open-label extension that allowed for the addition of nucleoside inhibitors of reverse transcriptase and dose escalation to maintain durability. The drug was well-tolerated and demonstrated robust antiviral activity, with demonstrable superiority of the 750 mg and 1000 mg three times daily regimens. Thirty subjects who continued to receive therapy at 12 months attained a persistent 1.6 log10 reduction in HIV RNA, accompanied by a mean increase in CD4 cells of 180-200/cu mm. Studies of viral genotype and phenotype after virus rebound revealed that the initial active site mutation allowing for nelfinavir resistance is mediated by a unique amino acid substitution in the HIV-1 protease D30N, which does not confer in vitro phenotypic cross-resistance to the currently available protease inhibitors.
PMID:9607830 Markowitz M et al; J Infect Dis 177 (6): 1533-40 (1998)
For more Therapeutic Uses (Complete) data for NELFINAVIR MESYLATE (6 total), please visit the HSDB record page.
In adults, the most frequent adverse effect associated with nelfinavir therapy is mild to moderate diarrhea.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 661
Rash has been reported in 13% of adults receiving nelfinavir in the recommended dosage in phase II/III clinical studies. Allergic reaction, dermatitis, folliculitis, fungal dermatitis, maculopapular rash, pruritus, sweating, and urticaria have occurred in less than 2% of adults receiving nelfinavir in clinical studies. Hypersensitivity reactions, including bronchospasm, moderate to severe rash, fever, and edema, possibly related to nelfinavir have been reported during postmarketing surveillance.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 661
In phase II/III clinical studies, asthenia occurred in 1% of adults receiving the usual dosage of nelfinavir in conjunction with 2 nucleoside reverse transcriptase inhibitors. Anxiety, depression, dizziness, emotional lability, headache (including migraine headache), hyperkinesia, insomnia,malaise, paresthesia, seizures, sleep disorders, somnolence, and suicidal ideation have been reported in less than 2% of adults receiving nelfinavir in clinical studies.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 661
Substantial increases in serum concentrations of AST (SGOT) or ALT (SGPT) (increase from normal baseline values to 5.1-10 times the usual normal value or increase from baseline values of 1.25-2.5 times the normal value to more than 10 times the usual normal value) occurred in up to 3% of adults receiving nelfinavir in clinical studies. Hepatitis, increases in serum alkaline phosphate concentrations, increases in Gamma-glutamyltransferase (GGT, GGTP) concentrations, or abnormal liver function test results have been reported in less than 2% of adults receiving nelfinavir in clinical studies.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 661
For more Drug Warnings (Complete) data for NELFINAVIR MESYLATE (19 total), please visit the HSDB record page.
Viracept is indicated in antiretroviral combination treatment of human-immunodeficiency-virus (HIV-1)-infected adults, adolescents and children of three years of age and older.
In protease-inhibitor (PI)-experienced patients, the choice of nelfinavir should be based on individual viral resistance testing and treatment history.
HIV Protease Inhibitors
Inhibitors of HIV PROTEASE, an enzyme required for production of proteins needed for viral assembly. (See all compounds classified as HIV Protease Inhibitors.)
J05AE04
Distribution of nelfinavir into body tissues and fluids has not been fully characterized. The volume of distribution of nelfinavir following oral administration in animals is 27 L/kg, suggesting extensive tissue distribution. Studies in rats indicate that, at 4 hours after oral administration of radiolabeled nelfinavir, concentrations of the drug in liver, lymph nodes, pancreas, kidney, lungs, submaxillary glands, heart, and spleen exceed concurrent plasma concentrations. Nelfinavir has been detected in brain tissue in rats.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 667
Nelfinavir is greater than 98% bound to plasma proteins, mostly to albumin and alpha1-acid glycoprotein. It is present in the CSF at less than 1% of plasma concentrations, at least in part due to its extensive binding to plasma protein but perhaps also due to the P-glycoprotein at the blood-brain barrier.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1370
Nelfinavir and its metabolites are eliminated primarily in feces, with less than 2% of the drug being excreted in the urine. Moderate or severe liver disease may prolong the half-life and increase plasma concentrations of the parent drug while lowering plasma concentrations of M8 /(a major hydroxy-t-butylamide metabolite)/.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1370
Nelfinavir absorption is very sensitive to food effects; a moderate fat meal increases the AUC 2 to 3 fold, and higher concentrations are achieved with high fat meals.
Brunton, L. Chabner, B, Knollman, B. Goodman and Gillman's The Pharmaceutical Basis of Therapeutics, Twelth Edition, McGraw Hill Medical, New York, NY. 2011, p. 1654
For more Absorption, Distribution and Excretion (Complete) data for NELFINAVIR MESYLATE (8 total), please visit the HSDB record page.
Nelfinavir undergoes oxidative metabolism in the liver primarily by CYP3A4, but also by CYP2C19 and CYP2D6. Its major hydroxy-t-butylamide metabolite (M8) has in vitro antiretroviral activity comparable to that of the parent drug but achieves plasma levels that are only 40% of nelfinavir levels. The M8 metabolite is generated primarily by CYP2C19.
Brunton, L. Chabner, B, Knollman, B. Goodman and Gillman's The Pharmaceutical Basis of Therapeutics, Twelth Edition, McGraw Hill Medical, New York, NY. 2011, p. 1655
The plasma elimination half-life of nelfinavir in individuals 13 years of age and older is 3.5-5 hours.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 667
The HIV-1 protease inhibitor nelfinavir (NFV) has off-target effects upon host enzymes, including inhibition of the 20S proteasome, resulting in activation of PP1. HIV-1-associated monocyte/macrophage activation, in part a result of systemically elevated levels of microbial products including LPS, is associated with risk of mortality, independent of viremia or CD4 T cell loss. This study tested the hypothesis that activation of protein phosphatases by NFV would reduce activation of monocytes/macrophages through dephosphorylation of signal transduction proteins. NFV uniquely blocked LPS-induced production by human monocyte-derived macrophages of the inflammatory cytokines TNF and IL-6, as well as sCD14. Although NFV failed to modulate NF-?B, NFV treatment reduced phosphorylation of AKT and MAPKs. Inhibition of PP2 with okadaic acid blocked the anti-inflammatory effect of NFV, whereas the PP1 inhibitor calyculin A failed to counter the anti-inflammatory effects of NFV. For in vivo studies, plasma sCD14 and LPS were monitored in a cohort of 31 pediatric HIV-1 patients for over 2 years of therapy. Therapy, including NFV, reduced sCD14 levels significantly compared with IDV or RTV, independent of ?LPS levels, VL, CD4 T cell frequency, or age. The hypothesis was supported as NFV induced activation of PP2 in macrophages, resulting in disruption of inflammatory cell signaling pathways. In vivo evidence supports that NFV may offer beneficial effects independent of antiviral activity by reducing severity of chronic innate immune activation in HIV-1 infection.
PMID:22786868 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3441314 Wallet MA et al; J Leukoc Biol 92 (4): 795-805 (2012)
While the complete mechanism(s) of antiviral activity of nelfinavir has not been fully elucidated, nelfinavir apparently inhibits replication of human immunodeficiency virus type 1 (HIV-1) and type 2 (HIV-2) by interfering with HIV protease. The drug, therefore, exerts a virustatic effect against retroviruses by acting as an HIV protease inhibitor.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 666
Nelfinavir inhibits protease by reversibly binding to the active site, preventing polypeptide processing and subsequent viral maturation. Viral particles produced in the presence of nelfinavir are immature and noninfectious.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1370
Unlike nucleoside reverse transcriptase inhibitors, the antiretroviral activity of nelfinavir does not depend on intracellular conversion to an active metabolite. Nelfinavir and other HIV protease inhibitors (e.g., amprenavir, indinavir, lopinavir, ritonavir, saquinavir) act at a different stage of the HIV replication cycle than other currently available antiretroviral agents, including nucleoside reverse transcriptase inhibitors and nonnucleoside reverse transcriptase inhibitors. Results of in vitro studies indicate that the antiretroviral effects of nelfinavir and nucleoside antiretroviral agents may be additive (didanosine or stavudine) or synergistic (lamivudine, zalcitabine, zidovudine). In vitro studies evaluating the antiretroviral effects of nelfinavir used with other HIV protease inhibitors (amprenavir, indinavir, ritonavir, saquinavir) has resulted in variable results ranging from antagonism to synergism.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 666
Nelfinavir is a selective, competitive, reversible inhibitor of HIV protease. HIV protease, an aspartic endopeptidase that functions as a homodimer, plays an essential role in the HIV replication cycle and the formation of infectious virus. During HIV replication, HIV protease cleaves viral polypeptide products of the gag and gag-pol genes (i.e., p55 and p160) to form structural proteins of the virion core (i.e., p17, p24, p9, and p7) and essential viral enzymes (i.e., reverse transcriptase, integrase, and protease). By interfering with the formation of these essential proteins and enzymes, nelfinavir blocks maturation of the virus and causes the formation of nonfunctional, immature, noninfectious virions. Nelfinavir is active in both acutely and chronically infected cells since it targets the HIV replication cycle after translation and before assembly. Thus, the drug is active in chronically infected cells (e.g., monocytes, macrophages) that generally are not affected by nucleoside reverse transcriptase inhibitors (e.g., abacavir, didanosine, lamivudine, stavudine, zalcitabine, zidovudine). Nelfinavir does not affect early stages of the HIV replication cycle; however, the drug interferes with the production of infectious HIV and limits further infectious spread of the virus.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 666