

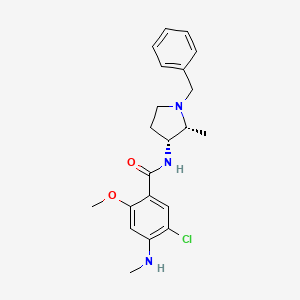

1. Cis-n-(1-benzyl-2-methylpyrrolidin-3-yl)-5-chloro-2-methoxy-4-methylaminobenzamide
2. Emonapride
3. Nemonapride, (cis)-isomer
4. Ym 09151-2
5. Ym 09151-m
6. Ym-09151-2
1. Emonapride
2. Emilace
3. [3h]nemonapride
4. 75272-39-8
5. (+)-nemonapride
6. (2r,3r)-nemonapride
7. N-[(2r,3r)-1-benzyl-2-methylpyrrolidin-3-yl]-5-chloro-2-methoxy-4-(methylamino)benzamide
8. Chebi:64219
9. Q88t5p3444
10. N-(cis-1-benzyl-2-methylpyrrolidin-3-yl)-5-chloro-2-methoxy-4-(methylamino)benzamide
11. 187139-87-3
12. Nemonapride [inn:jan]
13. Ym-09151
14. Unii-q88t5p3444
15. Emilace (tn)
16. Ym-09151-2
17. Nemonapride (jan/inn)
18. Nemonapride [mi]
19. Nemonapride [inn]
20. Nemonapride [jan]
21. (+-)-cis-n-(1-benzyl-2-methyl-3-pyrrolidinyl)-5-chloro-4-(methylamino)-o-anisamide
22. (+-)-cis-n-(1-benzyl-2-methylpyrrolidin-3-yl)-5-chloro-2-methoxy-4-methylaminobenzamide
23. N-(2rs,3rs)-(1-benzyl-2-methyl-3-pyrrolidinyl)-5-chloro-2-methoxy-4-methylaminobenzamide
24. Nemonapride [mart.]
25. Gtpl962
26. Nemonapride [who-dd]
27. Schembl1649724
28. Chembl2261102
29. Dtxsid601116422
30. Zinc538069
31. Bdbm50487259
32. Pdsp1_001527
33. Pdsp2_001511
34. Ncgc00371107-01
35. Benzamide, 5-chloro-2-methoxy-4-(methylamino)-n-((2r,3r)-2-methyl-1-(phenylmethyl)-3-pyrrolidinyl)-, Rel-
36. Benzamide, 5-chloro-2-methoxy-4-(methylamino)-n-(2-methyl-1-(phenylmethyl)-3-pyrrolidinyl)-, Cis-(+-)-
37. Hy-103415
38. B6843
39. Cs-0027847
40. D01468
41. Brd-k47289124-001-02-6
42. (+/-)-cis-n-(1-benzyl-2-methyl-3-pyrrolidinyl)-5-chloro-4-(methylamino)-o-anisamide
43. 5-chloro-2-methoxy-4-(methylamino)-n-[(2r,3r)-2-methyl-1-(phenylmethyl)-3-pyrrolidinyl]benzamide
44. 5-chloro-2-methoxy-4-methylamino-n-[(2r,3r)-2-methyl-1-(phenylmethyl)pyrrolidin-3-yl]benzamide
45. Benzamide, 5-chloro-2-methoxy-4-(methylamino)-n-(2-methyl-1- (phenylmethyl)-3-pyrrolidinyl)-, Cis-(+/-)-
| Molecular Weight | 387.9 g/mol |
|---|---|
| Molecular Formula | C21H26ClN3O2 |
| XLogP3 | 3.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Exact Mass | 387.1713548 g/mol |
| Monoisotopic Mass | 387.1713548 g/mol |
| Topological Polar Surface Area | 53.6 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 486 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antipsychotic Agents
Agents that control agitated psychotic behavior, alleviate acute psychotic states, reduce psychotic symptoms, and exert a quieting effect. They are used in SCHIZOPHRENIA; senile dementia; transient psychosis following surgery; or MYOCARDIAL INFARCTION; etc. These drugs are often referred to as neuroleptics alluding to the tendency to produce neurological side effects, but not all antipsychotics are likely to produce such effects. Many of these drugs may also be effective against nausea, emesis, and pruritus. (See all compounds classified as Antipsychotic Agents.)
Dopamine Antagonists
Drugs that bind to but do not activate DOPAMINE RECEPTORS, thereby blocking the actions of dopamine or exogenous agonists. Many drugs used in the treatment of psychotic disorders (ANTIPSYCHOTIC AGENTS) are dopamine antagonists, although their therapeutic effects may be due to long-term adjustments of the brain rather than to the acute effects of blocking dopamine receptors. Dopamine antagonists have been used for several other clinical purposes including as ANTIEMETICS, in the treatment of Tourette syndrome, and for hiccup. Dopamine receptor blockade is associated with NEUROLEPTIC MALIGNANT SYNDROME. (See all compounds classified as Dopamine Antagonists.)