
1. Ar-13324
1. 1254032-66-0
2. Ar-11324 Free Base
3. Rhopressa
4. Netarsudil [usan]
5. Netarsudil Free Base
6. W6i5qdt7qi
7. Ar-13324
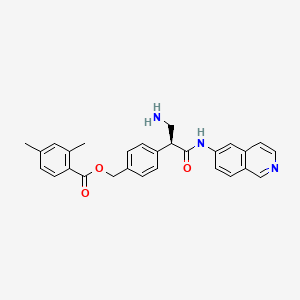
8. [4-[(2s)-3-amino-1-(isoquinolin-6-ylamino)-1-oxopropan-2-yl]phenyl]methyl 2,4-dimethylbenzoate
9. (4-((1s)-1-(aminomethyl)-2-(isoquinolin-6-ylamino)-2-oxoethyl)phenyl)methyl 2,4- Dimethylbenzoate
10. Benzoic Acid, 2,4-dimethyl-, (4-((1s)-1-(aminomethyl)-2-(6-isoquinolinylamino)-2-oxoethyl)phenyl)methyl Ester
11. Rhokiinsa
12. Unii-w6i5qdt7qi
13. Netarsudil [mi]
14. Netarsudil [inn]
15. Netarsudil (usan/inn)
16. Netarsudil [usan:inn]
17. Netarsudil [who-dd]
18. Gtpl9322
19. Chembl4594250
20. Schembl16036278
21. Dtxsid001027774
22. Bdbm50546247
23. Zinc113149554
24. Ar11324
25. Db13931
26. Ac-31227
27. Ar-11324
28. Ester 60 [pmid: 27072905]
29. D11030
30. Q27292390
| Molecular Weight | 453.5 g/mol |
|---|---|
| Molecular Formula | C28H27N3O3 |
| XLogP3 | 4.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 8 |
| Exact Mass | 453.20524173 g/mol |
| Monoisotopic Mass | 453.20524173 g/mol |
| Topological Polar Surface Area | 94.3 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 678 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Netarsudil is indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
FDA Label
Reduction of elevated intraocular pressure (IOP) in adult patients with primary open-angle glaucoma or ocular hypertension.
Aqueous humour flows out of the eye via two pathways: 1) the conventional trabecular pathway and 2) the unconventional uveoscleral pathway. And, although it has been shown that the conventional trabecular pathway accounts for most aqueous outflow due to various pathologies, most medications available for treating glaucoma target the uveoscleral pathway for treatment and leave the diseased trabecular pathway untreated and unhindered in its progressive deterioration and dysfunction. Netarsudil is subsequently a novel glaucoma medication that is both a rho kinase and norepinephrine transport (NATs)s inhibitor that specifically targets and inhibits rho kinase and NATS found in the conventional trabecular pathway while many of its contemporaries offer therapy that focuses on cell and muscle tissue remodelling
S01EX05
S - Sensory organs
S01 - Ophthalmologicals
S01E - Antiglaucoma preparations and miotics
S01EX - Other antiglaucoma preparations
S01EX05 - Netarsudil
Absorption
The systemic exposure of netarsudil and its active metabolite, AR-13503, after topical ocular administration of netarsudil opthalmic solution 0.02% once daily (one drop bilaterally in the morning) for eight days in 18 healthy subjects demonstrated no quantifiable plasma concentrations of netarsudil (lower limit of quantitation [LLOQ] 0.100 ng/mL) post dose on Day 1 and Day 8. Only one plasma concentration at 0.11 ng/mL for the active metabolite was observed for one subject on Day 8 at 8 hours post dose.
Route of Elimination
Clinical studies assessing the *in vitro* metabolism of netarsudil using corneal tissue from humans, human plasma, and human liver microsomes and microsomal S9 fractions demonstrated that netarsudil metabolism occurs through esterase activity. Subsequent metabolism of netarsudil's esterase metabolite, AR-13503, was not detectable. In fact, esterase metabolism in human plasma was not detected during a 3 hour incubation.
Volume of Distribution
As netarsudil and its active metabolite demonstrate a high degree of protein binding, it is expected to exhibit a low volume of distribution.
Clearance
The clearance of netarsudil is strongly influenced by its low plasma concetrations following topical administration and absorption and high protein binding in human plasma inn.
After topical ocular dosing, netarsudil is metabolized by esterases in the eye to its active metabolite, netarsudil-M1 (or AR-13503).
The half-life of netarsudil incubated *in vitro
with human corneal tissue is 175 minutes.
The medical condition glaucoma is a leading cause of progressive visual impairment and blindness across the world with primary open-angle glaucoma (POAG) being the major type of glaucoma. Elevated intraocular pressure (IOP) resulting from increased resistance to aqueous humor outflow is considered a major risk for the development and progression of POAG, but various clinical studies have demonstrated that the reduction and tight control of IOP can delay or prevent POAG and the vision loss associated with it. Ordinary physiological IOP results from aqueous humor produced by the ocular ciliary body and its outflow through two main outflow pathways: the conventional (trabecular) and the unconventional (uveoscleral) pathways. Under ordinary physiological conditions, diagnostic tracers have shown that the conventional trabecular pathway accounts for up to 90% of aqueous humor outflow. Through this pathway, aqueous humor drains from the anterior chamber sequentially through the uveal and corneoscleral meshwork beams, juxtacanalicular connective tissue (JCT) region, and inner wall (IW) endothelial cells of Schlemm's canal (SC) until finally entering the lumen of SC. From there aqueous humor drains into the collector channels, intravascular plexus, epscleral veins, and finally into the blood circulation. In glaucomatous eyes, elevated IOP is the result of abnormally increased resistance to aqueous outflow in the conventional trabecular pathway due to apparent increases in the contractile tone and stiffness of the trabecular pathway meshwork (TM), changes in extracellular matrix composition, and/or a decrease in the conductance of the IW endothelial cells of SC. Subsequently, as a rho kinase inhibitor, the novelty of netarsudil lies in its ability or specificity to apply its mechanism of action directly and specifically at the diseased TM of the conventional trabecular outflow pathway. In particular, rho kinases are serine/threonine kinases that function as important downstream effectors of Rho GTPase. Such activity in the TM and SC drives actomysin contraction, promotes extracellular matrix production, and increases cell stiffness. Acting as an inhibitor of rho kinase, netarsudil consequently reduces cell contraction, decreases the expression of fibrosis-related proteins, and reduces cell stiffness in the TM and SC cells. As a result, netarsudil has been able to demonstrate increases in trabecular outflow facility, increases in the effective filtration area of the TM, cause expansion of the TM tissue, and dilate episcleral veins. Furthermore, netarsudil is also believed to possess inhibitory action against the norepinephrine transporter (NET). Such inhibition of the NET prevents reuptake of norepinephrine at noradrenergic synapses, which results in an increase in the strength and duration of endogenous norepinephrine signaling. As a consequence of this enhanced signaling, norepinephrine-induced vasoconstriction that can reduce blood flow to the ciliary body may subsequently be responsible for a mechanism in which the formation of aqueous humor may be delayed, prolonged, or reduced as well.