


1. Circo Maren
2. Circo-maren
3. Ergobel
4. F.i. 6714
5. Fisifax
6. Nicergobeta
7. Nicergolin Atid
8. Nicergolin Lindo
9. Nicergolin Neuraxpharm
10. Nicergolin Ratiopharm
11. Nicergolin Teva
12. Nicergolin Von Ct
13. Nicergolin-neuraxpharm
14. Nicergolin-ratiopharm
15. Nicergolin-teva
16. Nicerium
17. Nicotergoline
18. Nimergoline
19. Sermion
20. Von Ct, Nicergolin
1. 27848-84-6
2. Nimergoline
3. Ergobel
4. Nicergolinum
5. Nicotergoline
6. Sermion
7. Cergodum
8. Duracebrol
9. Nicergolent
10. Vasospan
11. Circo-maren
12. Nimergoline Base
13. Dilasenil
14. Ergotop
15. Nilogrin
16. Memoq
17. Sermion (tn)
18. 10-methoxy-1,6-dimethylergoline-8beta-methanol 5-bromonicotinate
19. Jcv8365fwn
20. Chebi:31902
21. Nicergolin [german]
22. Nicergolina [dcit]
23. Nsc-150531
24. ((6ar,9r,10as)-10a-methoxy-4,7-dimethyl-4,6,6a,7,8,9,10,10a-octahydroindolo[4,3-fg]quinolin-9-yl)methyl 5-bromonicotinate
25. Fi-6714
26. Rp 19651
27. Ncgc00017259-04
28. Nicergolinum [inn-latin]
29. Nicergolina
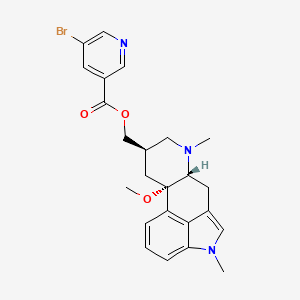
30. Nicergolin
31. Dsstox_cid_25607
32. Dsstox_rid_81000
33. Dsstox_gsid_45607
34. [(2s,4r,7r)-2-methoxy-6,11-dimethyl-6,11-diazatetracyclo[7.6.1.0^{2,7}.0^{12,16}]hexadeca-1(16),9,12,14-tetraen-4-yl]methyl 5-bromopyridine-3-carboxylate
35. [(6ar,9r,10as)-10a-methoxy-4,7-dimethyl-6a,8,9,10-tetrahydro-6h-indolo[4,3-fg]quinoline-9-yl]methyl 5-bromopyridine-3-carboxylate
36. Cas-27848-84-6
37. Fi 6714
38. Sr-01000597616
39. Einecs 248-694-6
40. Unii-jcv8365fwn
41. Nsc 150531
42. Brn 4828393
43. 1-methyl-lumilysergol 8-(5-bromonicotinate) 10-methyl Ether
44. Nicergoline,(s)
45. (+)-10-methoxy-1,6-dimethylergoline-8-beta-methanol 5-bromonicotinate
46. 10-methoxy-1,6-dimethylergoline-8beta-methanol 5-bromonicotinate (ester)
47. Nicergoline [usan:inn:ban:jan]
48. 10-methoxy-1,6-dimethyl-ergolin-8-beta-methanol-(5-bromnicotinat) [german]
49. 8-beta-((5-bromonicotinoyloxy)methyl)-1,6-dimethyl-10-alpha-methoxyergoline
50. Nicergoline- Bio-x
51. Mfcd00869626
52. 10-methoxy-1,6-dimethylergoline-8-methanol 5-bromo-3-pyrindinecarboxylate (ester)
53. Ergoline-8-beta-methanol, 10-methoxy-1,6-dimethyl-, 5-bromo-3-pyridinecarboxylate (ester)
54. Spectrum_001370
55. Nicergoline [mi]
56. 10-methoxy-1,6-dimethyl-ergolin-8-beta-methanol-(5-bromnicotinat)
57. Prestwick0_000147
58. Prestwick1_000147
59. Prestwick2_000147
60. Prestwick3_000147
61. Spectrum2_001414
62. Spectrum3_001933
63. Spectrum4_000440
64. Spectrum5_001352
65. Nicergoline [inn]
66. Nicergoline [jan]
67. 5-bromonicotinic Acid 10-methoxy-1,6-dimethylergoline-8-methyl Ester
68. Nicergoline [usan]
69. Ergoline-8-beta-methanol, 10-methoxy-1,6-dimethyl-, 5-bromonicotinate (ester)
70. Ergoline-8beta-methanol, 10-methoxy-1,6-dimethyl-, 5-bromonicotinate (ester)
71. Ergoline-8-methanol, 10-methoxy-1,6-dimethyl-, (8beta)-, 5-bromo-3-pyridinecarboxylate (ester)
72. Ergoline-8-methanol, 10-methoxy-1,6-dimethyl-, 5-bromo-3-pyridinecarboxylate (ester), (8beta)-
73. Nicergoline [mart.]
74. Schembl22964
75. Bspbio_000254
76. Bspbio_003533
77. Kbiogr_000800
78. Kbioss_001850
79. Nicergoline [who-dd]
80. Divk1c_000124
81. Spectrum1501133
82. Spbio_001488
83. Spbio_002193
84. Bpbio1_000280
85. Chembl1372950
86. Dtxsid7045607
87. Hms500g06
88. Kbio1_000124
89. Kbio2_001850
90. Kbio2_004418
91. Kbio2_006986
92. Kbio3_002787
93. Nicergoline (jp17/usan/inn)
94. Nicergoline For System Suitability
95. Ninds_000124
96. Hms1568m16
97. Hms1923o09
98. Hms2089i03
99. Hms2092f07
100. Hms2095m16
101. Hms3712m16
102. Hms3886c03
103. Nicergoline [ep Monograph]
104. Nicergoline For Peak Identification
105. Pharmakon1600-01501133
106. 4-oxazolecarboxylicacid, 5-methyl-
107. Ex-a4135
108. Hy-b0702
109. Zinc3873817
110. Tox21_110810
111. Ccg-39032
112. Nsc757853
113. S4797
114. Akos005067888
115. Akos015969107
116. Tox21_110810_1
117. Db00699
118. Nicergoline 1.0 Mg/ml In Acetonitrile
119. Nsc-757853
120. Idi1_000124
121. Qtl1_000060
122. Ncgc00017259-03
123. Ncgc00017259-05
124. Ncgc00017259-07
125. Ncgc00024678-02
126. Ncgc00024678-03
127. As-12239
128. Bn166697
129. Sbi-0051652.p002
130. N0904
131. D01290
132. T72213
133. Ab00052214-04
134. Ab00052214_05
135. 848n846
136. A853601
137. Sr-05000001751
138. J-016896
139. Nicergoline, Analytical Standard, For Drug Analysis
140. Q2623398
141. Sr-01000597616-1
142. Sr-01000597616-2
143. Sr-05000001751-1
144. Brd-k76810206-001-05-7
145. Brd-k76810206-001-06-5
146. Nicergoline, European Pharmacopoeia (ep) Reference Standard
147. (8beta)-10-methoxy-1,6-dimethylergoline-8-methanol 5-bromo-3-pyridinecarboxylate (ester)
148. [(8r,10s)-10-methoxy-1,6-dimethylergolin-8-yl]methyl 5-bromopyridine-3-carboxylate
149. 10-methoxy-1,6-dimethylergoline-8.beta.-methanol 5-bromonicotinate (ester)
150. Nicergoline For Peak Identification, European Pharmacopoeia (ep) Reference Standard
151. Nicergoline For System Suitability, European Pharmacopoeia (ep) Reference Standard
152. ((6ar,9r,10as)-10a-methoxy-4,7-dimethyl-4,6,6a,7,8,9,10,10a-octahydroindolo[4,3-fg]quinolin-9-yl)methyl5-bromonicotinate
153. [(6ar,9r,10as)-10a-methoxy-4,7-dimethyl-6a,8,9,10-tetrahydro-6h-indolo[4,3-fg]quinolin-9-yl]methyl 5-bromopyridine-3-carboxylate
154. 5-bromopyridine-3-carboxylic Acid [(8r,10s)-10-methoxy-1,6-dimethylergolin-8-yl]methyl Ester
155. Ergoline-8-methanol, 10-methoxy-1,6-dimethyl-, (8.beta.)-, 5-bromo-3-pyridinecarboxylate (ester)
156. Ergoline-8-methanol, 10-methoxy-1,6-dimethyl-,5-bromo-3-pyridinecarboxylate (ester), (8b)-
| Molecular Weight | 484.4 g/mol |
|---|---|
| Molecular Formula | C24H26BrN3O3 |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 5 |
| Exact Mass | 483.11575 g/mol |
| Monoisotopic Mass | 483.11575 g/mol |
| Topological Polar Surface Area | 56.6 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 681 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of senile dementia, migraines of vascular origin, transient ischemia, platelet hyper-aggregability, and macular degeneration.
Nicergoline is a potent vasodilator (improves brain blood flow). On the cerebral level it prompts a lowering of vascular resistance, an increase in arterial flow and stimulates the use of oxygen and glucose. Nicergoline also improves blood circulation in the lungs and limbs and has been shown to inhibit blood platelet aggregation.
Adrenergic alpha-Antagonists
Drugs that bind to but do not activate alpha-adrenergic receptors thereby blocking the actions of endogenous or exogenous adrenergic agonists. Adrenergic alpha-antagonists are used in the treatment of hypertension, vasospasm, peripheral vascular disease, shock, and pheochromocytoma. (See all compounds classified as Adrenergic alpha-Antagonists.)
Nootropic Agents
Drugs used to specifically facilitate learning or memory, particularly to prevent the cognitive deficits associated with dementias. These drugs act by a variety of mechanisms. (See all compounds classified as Nootropic Agents.)
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
C - Cardiovascular system
C04 - Peripheral vasodilators
C04A - Peripheral vasodilators
C04AE - Ergot alkaloids
C04AE02 - Nicergoline
Nicergoline acts by inhibiting the postsynaptic alpha(1)-adrenoceptors on vascular smooth muscle. This inhibits the vasoconstrictor effect of circulating and locally released catecholamines (epinephrine and norepinephrine), resulting in peripheral vasodilation. Therefore the mechanism of Nicergoline is to increase vascular circulation in the brain, thereby enhancing the transmission of nerve signals across the nerve fibres, which secrete acetylcholine as a neural transmitter.