

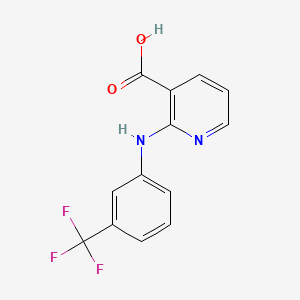

1. Acid, Niflumic
2. Donalgin
3. Flunir
4. Niflactol
5. Niflugel
6. Nifluril
1. 4394-00-7
2. Landruma
3. Nifluril
4. Forenol
5. Nifluminic Acid
6. Niflugel
7. Acido Niflumico
8. Acide Niflumique
9. Up 83
10. 2-{[3-(trifluoromethyl)phenyl]amino}pyridine-3-carboxylic Acid
11. Acide Niflumique [inn-french]
12. Acido Niflumico [inn-spanish]
13. Acidum Niflumicum [inn-latin]
14. 2-[3-(trifluoromethyl)anilino]pyridine-3-carboxylic Acid
15. 2-(3-(trifluoromethyl)anilino)nicotinic Acid
16. 2-(3-(trifluoromethyl)-phenyl)aminonicotinic Acid
17. 2-(3-trifluoromethylanilino)nicotinic Acid
18. 2-((3-(trifluoromethyl)phenyl)amino)nicotinic Acid
19. 2-[3-(trifluoromethyl)anilino]nicotinic Acid
20. 3-pyridinecarboxylic Acid, 2-[[3-(trifluoromethyl)phenyl]amino]-
21. Lopac-n-0630
22. 2-{[3-(trifluoromethyl)phenyl]amino}nicotinic Acid
23. 2-(3-(trifluoromethyl)phenylamino)nicotinic Acid
24. Niflumic Acid (inn)
25. Nsc-758196
26. 2-[(3-trifluoromethyl)phenyl]amino-3-pyridine-carboxylic Acid
27. 4u5mp5iud8
28. Mls000069713
29. Nfl
30. Chebi:34888
31. 3-pyridinecarboxylic Acid, 2-((3-(trifluoromethyl)phenyl)amino)-
32. Up-83
33. Ncgc00015724-10
34. Niflumate
35. Smr000058199
36. Cas-4394-00-7
37. Dsstox_cid_3368
38. 2-(3-[trifluoromethyl]anilino)nicotinic Acid
39. Acidum Niflumicum
40. Dsstox_rid_76996
41. Niflumic Acid [inn]
42. Dsstox_gsid_23368
43. Acide Niflumique [french]
44. Acido Niflumico [italian]
45. Niflamol
46. Niflugel (tn)
47. Sc 1332
48. Ccris 5740
49. Sr-01000000231
50. 2-(alpha,alpha,alpha-trifluoro-m-toluidino)nicotinic Acid
51. Einecs 224-516-2
52. 2-(3-trifluoromethyl-phenylamino)-nicotinic Acid
53. Unii-4u5mp5iud8
54. Brn 0489360
55. 2-[[3-(trifluoromethyl)phenyl]amino]pyridine-3-carboxylic Acid
56. Niflumic-acid
57. Ni-flumic Acid
58. Niflumic Acid [inn:ban:dcf]
59. Actol, Analgesic
60. Aza-2 Dimethyl-2',3' (tetrazolyl-5)-6 Diphenylamino [french]
61. Prestwick_890
62. Mfcd00010569
63. Aza-2 Dimethyl-2',3' (tetrazolyl-5)-6 Diphenylamino
64. Spectrum_001353
65. 1td7
66. Opera_id_1746
67. Prestwick0_000255
68. Prestwick1_000255
69. Prestwick2_000255
70. Prestwick3_000255
71. Spectrum2_000794
72. Spectrum3_001485
73. Spectrum4_000043
74. Spectrum5_001216
75. Niflumic Acid (hit 16)
76. N 0630
77. Niflumic Acid [mi]
78. 2-[(3-trifluoromethylphenyl)amino]nicotinic Acid
79. Cbiol_001828
80. Lopac0_000845
81. Schembl24706
82. Bspbio_000070
83. Bspbio_001393
84. Bspbio_003069
85. Kbiogr_000113
86. Kbiogr_000505
87. Kbioss_000113
88. Kbioss_001833
89. 5-22-13-00598 (beilstein Handbook Reference)
90. Mls001076327
91. Chembl63323
92. Divk1c_000277
93. Spectrum1502015
94. Spbio_000928
95. Spbio_002289
96. Niflumic Acid [mart.]
97. Bpbio1_000078
98. Gtpl2439
99. Niflumic Acid [who-dd]
100. Dtxsid1023368
101. Bdbm85507
102. Hms500n19
103. Kbio1_000277
104. Kbio2_000113
105. Kbio2_001833
106. Kbio2_002681
107. Kbio2_004401
108. Kbio2_005249
109. Kbio2_006969
110. Kbio3_000225
111. Kbio3_000226
112. Kbio3_002569
113. Ninds_000277
114. 2-[(3-trifluoromethyl)phenyl]amino]-3-pyridinecarboxylic Acid
115. Bio1_000114
116. Bio1_000603
117. Bio1_001092
118. Bio2_000113
119. Bio2_000593
120. Hms1361f15
121. Hms1568d12
122. Hms1791f15
123. Hms1921d12
124. Hms1989f15
125. Hms2090d19
126. Hms2095d12
127. Hms2234f11
128. Hms3262j11
129. Hms3374h01
130. Hms3402f15
131. Hms3649a08
132. Hms3656p14
133. Hms3712d12
134. Hms3885i03
135. Nicotinic Acid, 2-(alpha,alpha,alpha-trifluoro-m-toluidino)-
136. Pharmakon1600-01502015
137. Zinc125031
138. Hy-b0493
139. Niflumic Acid [ep Impurity]
140. Nsc_4488
141. Tox21_110206
142. Tox21_500845
143. Bbl003619
144. Ccg-40157
145. Dl-457
146. Nicotinic Acid, 2-(.alpha.,.alpha.,.alpha.-trifluoro-m-toluidino)-
147. Niflumic Acid [ep Monograph]
148. Nsc758196
149. S3018
150. Stk803109
151. Akos000519590
152. Tox21_110206_1
153. Ac-2652
154. Db04552
155. Gs-3202
156. Lp00845
157. Nsc 758196
158. Sdccgsbi-0050821.p004
159. Idi1_000277
160. Idi1_033863
161. Ncgc00015724-01
162. Ncgc00015724-02
163. Ncgc00015724-03
164. Ncgc00015724-04
165. Ncgc00015724-05
166. Ncgc00015724-06
167. Ncgc00015724-07
168. Ncgc00015724-08
169. Ncgc00015724-09
170. Ncgc00015724-11
171. Ncgc00015724-12
172. Ncgc00015724-13
173. Ncgc00015724-14
174. Ncgc00015724-17
175. Ncgc00015724-29
176. Ncgc00023636-03
177. Ncgc00023636-04
178. Ncgc00023636-05
179. Ncgc00023636-06
180. Ncgc00023636-07
181. Ncgc00023636-08
182. Ncgc00023636-09
183. Ncgc00261530-01
184. Cas_4394-00-7
185. Sbi-0050821.p003
186. 2(3'-trifluormethylanilino)-nicotinic Acid
187. Ab00052255
188. Am20070143
189. Eu-0100845
190. Ft-0603659
191. Niflumic Acid 100 Microg/ml In Acetonitrile
192. Sw197011-3
193. T2353
194. A23222
195. D08275
196. H10042
197. S00109
198. Ab00052255-17
199. Ab00052255_18
200. Ab00052255_19
201. 2-((3-(trifluoromethyl)phenyl)amino)nicotinicacid
202. 394n007
203. Q304285
204. Sr-01000000231-2
205. Sr-01000000231-5
206. Sr-01000000231-6
207. W-106215
208. Brd-k98763141-001-04-3
209. Brd-k98763141-001-06-8
210. Brd-k98763141-001-17-5
211. Sr-01000000231-11
212. 2-[3-(trifluoromethyl)anilino]-3-pyridinecarboxylic Acid
213. Niflumic Acid, European Pharmacopoeia (ep) Reference Standard
| Molecular Weight | 282.22 g/mol |
|---|---|
| Molecular Formula | C13H9F3N2O2 |
| XLogP3 | 3.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 3 |
| Exact Mass | 282.06161202 g/mol |
| Monoisotopic Mass | 282.06161202 g/mol |
| Topological Polar Surface Area | 62.2 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 349 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used in the treatment of rheumatoid arthritis.
Niflumic acid, a nonsteroidal anti-inflammatory fenamate, is a Ca2+-activated Cl- channel blocker.
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Cyclooxygenase Inhibitors
Compounds or agents that combine with cyclooxygenase (PROSTAGLANDIN-ENDOPEROXIDE SYNTHASES) and thereby prevent its substrate-enzyme combination with arachidonic acid and the formation of eicosanoids, prostaglandins, and thromboxanes. (See all compounds classified as Cyclooxygenase Inhibitors.)
M02AA17
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
M - Musculo-skeletal system
M01 - Antiinflammatory and antirheumatic products
M01A - Antiinflammatory and antirheumatic products, non-steroids
M01AX - Other antiinflammatory and antirheumatic agents, non-steroids
M01AX02 - Niflumic acid
M - Musculo-skeletal system
M02 - Topical products for joint and muscular pain
M02A - Topical products for joint and muscular pain
M02AA - Antiinflammatory preparations, non-steroids for topical use
M02AA17 - Niflumic acid
Absorption
Well absorbed following oral administration.
Hepatic.
2.5 hours
Niflumic acid is able to inhibit both phospholipase A2 as well as COX-2, thereby acting as an antiinflamatory and pain reduction agent.