

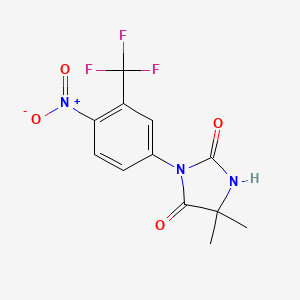

1. 5,5-dimethyl-3-(4-nitro-3-(trifluoromethyl)phenyl)- 2,4-imidazolidinedione
2. Anandron
3. Nilandron
4. Ru 23908
5. Ru 23908-10
6. Ru-23908
1. 63612-50-0
2. Anandron
3. Nilandron
4. 5,5-dimethyl-3-(4-nitro-3-(trifluoromethyl)phenyl)imidazolidine-2,4-dione
5. Ru-23908
6. Nilutamida
7. Nilutamidum
8. 5,5-dimethyl-3-[4-nitro-3-(trifluoromethyl)phenyl]imidazolidine-2,4-dione
9. Ru 23908
10. 5,5-dimethyl-3-(alpha,alpha,alpha-trifluoro-4-nitro-m-tolyl)hydantoin
11. Nilandrone
12. Nilandron;ru 23908
13. Nsc-758683
14. Chembl1274
15. Chebi:7573
16. Nilutamidum [latin]
17. Nilutamida [spanish]
18. 2,4-imidazolidinedione,5,5-dimethyl-3-[4-nitro-3-(trifluoromethyl)phenyl]-
19. 5,5-dimethyl-3-(4-nitro-3-(trifluoromethyl)phenyl)-2,4-imidazolidinedione
20. 5,5-dimethyl-3-[4-nitro-3-(trifluoromethyl)phenyl]-2,4-imidazolidinedione
21. 2,4-imidazolidinedione, 5,5-dimethyl-3-(4-nitro-3-(trifluoromethyl)phenyl)-
22. 51g6i8b902
23. Ncgc00015754-08
24. Cas-63612-50-0
25. Dsstox_cid_14165
26. Dsstox_rid_79118
27. Dsstox_gsid_34165
28. Nilutamide [usan:inn:ban]
29. 5,5-dimethyl-3-(4-nitro-3-(trifluoromethyl)-phenyl)imidazolidine-2,4-dione
30. Nilandron (tn)
31. Ru 23908-10
32. Sr-01000076034
33. Nilutamide (usan/inn)
34. Brn 0841906
35. Unii-51g6i8b902
36. Nilutamide, Solid
37. 2,4-imidazolidinedione, 5,5-dimethyl-3-[4-nitro-3-(trifluoromethyl)phenyl]-
38. 1-(3'-trifluoromethyl-4'-nitrophenyl)-4,4-dimethylimidazolidine-2,5-dione
39. Mfcd00864670
40. Spectrum_001625
41. Tocris-1759
42. Nilutamide [mi]
43. Specplus_000902
44. Nilutamide [inn]
45. Prestwick0_000928
46. Prestwick1_000928
47. Prestwick2_000928
48. Prestwick3_000928
49. Spectrum2_001973
50. Spectrum3_001633
51. Spectrum4_000600
52. Spectrum5_001512
53. Lopac-n-8534
54. Nilutamide [usan]
55. Nilutamide [vandf]
56. N 8534
57. Nilutamide [mart.]
58. Nilutamide [usp-rs]
59. Nilutamide [who-dd]
60. Bidd:pxr0177
61. Lopac0_000879
62. Schembl12670
63. Bspbio_000836
64. Bspbio_003325
65. Kbiogr_001100
66. Kbioss_002105
67. Mls002154066
68. Bidd:gt0683
69. Divk1c_006998
70. Spectrum1504152
71. Spbio_002125
72. Spbio_003015
73. Bpbio1_000920
74. Gtpl2864
75. Dtxsid3034165
76. Nilutamide [orange Book]
77. Kbio1_001942
78. Kbio2_002105
79. Kbio2_004673
80. Kbio2_007241
81. Kbio3_002545
82. Nilutamide [ep Monograph]
83. Bcpp000148
84. Hms1570j18
85. Hms1922f03
86. Hms2093a10
87. Hms2097j18
88. Hms2230e03
89. Hms3262p19
90. Hms3268c18
91. Hms3369i02
92. Hms3414n15
93. Hms3678n13
94. Hms3714j18
95. Nilutamide [usp Monograph]
96. Pharmakon1600-01504152
97. Amy32529
98. Bcp26617
99. Zinc3874498
100. Tox21 110213
101. Tox21_110213
102. Tox21_301589
103. Tox21_500879
104. Bdbm50135912
105. Ccg-39427
106. Nsc758683
107. S4836
108. Stk633161
109. Akos005565152
110. Akos025147305
111. Tox21_110213_1
112. Ac-5260
113. Bcp9000990
114. Db00665
115. Lp00879
116. Nsc 758683
117. Ru23908
118. Sb19036
119. Sdccgsbi-0050854.p004
120. 5,5-dimethyl-3-[4-nitro-3-(trifluoromethyl)phenyl]-1,3-diazolidine-2,4-dione
121. Ncgc00015754-01
122. Ncgc00015754-02
123. Ncgc00015754-03
124. Ncgc00015754-04
125. Ncgc00015754-05
126. Ncgc00015754-06
127. Ncgc00015754-07
128. Ncgc00015754-09
129. Ncgc00015754-10
130. Ncgc00015754-11
131. Ncgc00015754-12
132. Ncgc00015754-15
133. Ncgc00015754-16
134. Ncgc00015754-22
135. Ncgc00025280-01
136. Ncgc00025280-02
137. Ncgc00025280-03
138. Ncgc00025280-04
139. Ncgc00025280-05
140. Ncgc00025280-06
141. Ncgc00025280-07
142. Ncgc00025280-08
143. Ncgc00255271-01
144. Ncgc00261564-01
145. As-14123
146. Bn166184
147. Hy-13702
148. Smr001233381
149. Sbi-0050854.p003
150. Ab00053180
151. Cs-0007719
152. Eu-0100879
153. Ft-0630740
154. N1212
155. C08164
156. D00965
157. Ab00053180_07
158. 612n500
159. A834440
160. L000759
161. Q3877030
162. Ru-23908;ru 23908;ru23908
163. Sr-01000076034-1
164. Sr-01000076034-3
165. Sr-01000076034-5
166. Sr-01000076034-6
167. Sr-01000076034-9
168. Brd-k23566484-001-05-2
169. Brd-k23566484-001-09-4
170. Z2417927201
171. Nilutamide, European Pharmacopoeia (ep) Reference Standard
172. 1-(3'-trifluoromethyl-4'-nitrophenyl)-4,4-dimethyl-imidazoline-2,5-dione
173. 1-(3'-trifluoromethyl-4'-nitrophenyl)4,4-dimethyl-imidazoline-2,5-dione
174. 1-(3'-trifluoromethyl-4'nitrophenyl)-4,4-dimethyl-imidazoline-2,5-dione
175. 1-(3'trifluoromethyl-4'-nitropheyl)-4,4-dimethyl-imidazoline-2,5-dione
176. 1-(3-'trifluoromethyl-4'-nitrophenyl)-4,4-dimethyl-imidazoline-2,5-dione
177. 1-(3-trifluoromethyl-4-nitro-phenyl)-4,4-dimethyl Imidazolidine-2,5-dione
178. 1-(3-trifluoromethyl-4-nitro-phenyl)-4,4-dimethyl-imidazolidine-2,5-dione
179. 1-(3-trifluoromethyl-4-nitro-phenyl)-4,4-dimethyl-imidazoline-2,5-dione
180. 1-(3-trifluoromethyl-4-nitrophenyl)-4,4-dimethyl-imidazoline-2,5-dione
181. 3-(3-(trifluoromethyl)-4-nitrophenyl)-5,5-dimethylimidazolidine-2,4-dione
182. 5,5-dimethyl-3-(4-nitro-3-trifluoromethyl-phenyl)-imidazolidine-2,4-dione
183. Diethyl1,4-dihydro-2,6-dimethyl-1,4-diphenyl-3,5-pyridinedicarboxylate
184. 5,5-dimethyl-3-(.alpha.,.alpha.,.alpha.-trifluoro-4-nitro-m-tolyl)hydantoin
185. 5,5-dimethyl-3-[4-nitro-3-(trifluoromethyl)phenyl]-imidazolidine-2,4-dione
| Molecular Weight | 317.22 g/mol |
|---|---|
| Molecular Formula | C12H10F3N3O4 |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 1 |
| Exact Mass | 317.06234029 g/mol |
| Monoisotopic Mass | 317.06234029 g/mol |
| Topological Polar Surface Area | 95.2 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 515 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Nilandron |
| PubMed Health | Nilutamide (By mouth) |
| Drug Classes | Antiandrogen |
| Drug Label | NILANDRON tablets contain nilutamide, a nonsteroidal, orally active antiandrogen having the chemical name 5,5-dimethyl-3-[4-nitro-3-(trifluoromethyl)phenyl]-2,4-imidazolidinedione with the following structural formula:Nilutamide is a microcrystalli... |
| Active Ingredient | Nilutamide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 150mg |
| Market Status | Prescription |
| Company | Covis Pharma Sarl |
| 2 of 2 | |
|---|---|
| Drug Name | Nilandron |
| PubMed Health | Nilutamide (By mouth) |
| Drug Classes | Antiandrogen |
| Drug Label | NILANDRON tablets contain nilutamide, a nonsteroidal, orally active antiandrogen having the chemical name 5,5-dimethyl-3-[4-nitro-3-(trifluoromethyl)phenyl]-2,4-imidazolidinedione with the following structural formula:Nilutamide is a microcrystalli... |
| Active Ingredient | Nilutamide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 150mg |
| Market Status | Prescription |
| Company | Covis Pharma Sarl |
For use in combination with surgical castration for the treatment of metastatic prostate cancer involving distant lymph nodes, bone, or visceral organs (Stage D2).
FDA Label
Nilutamide is an antineoplastic hormonal agent primarily used in the treatment of prostate cancer. Nilutamide is a pure, nonsteroidal anti-androgen with affinity for androgen receptors (but not for progestogen, estrogen, or glucocorticoid receptors). Consequently, Nilutamide blocks the action of androgens of adrenal and testicular origin that stimulate the growth of normal and malignant prostatic tissue. Prostate cancer is mostly androgen-dependent and can be treated with surgical or chemical castration. To date, antiandrogen monotherapy has not consistently been shown to be equivalent to castration. The relative binding affinity of nilutamide at the androgen receptor is less than that of bicalutamide, but similar to that of hydroxuflutamide.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Androgen Antagonists
Compounds which inhibit or antagonize the biosynthesis or actions of androgens. (See all compounds classified as Androgen Antagonists.)
L - Antineoplastic and immunomodulating agents
L02 - Endocrine therapy
L02B - Hormone antagonists and related agents
L02BB - Anti-androgens
L02BB02 - Nilutamide
Absorption
Rapidly and completely absorbed, yielding high and persistent plasma concentrations.
Route of Elimination
Nilutamide is extensively metabolized andless than 2% of the drug is excreted unchanged in urine after 5 days. Fecal elimination is negligible, ranging from 1.4% to 7% of the dose after 4 to 5 days.
The results of a human metabolism study using 14C-radiolabelled tablets show that nilutamide is extensively metabolized and less than 2% of the drug is excreted unchanged in urine after 5 days.
38.0-59.1 hours
Nilutamide competes with androgen for the binding of androgen receptors, consequently blocking the action of androgens of adrenal and testicular origin that stimulate the growth of normal and malignant prostatic tissue. This blockade of androgen receptors may result in growth arrest or transient tumor regression through inhibition of androgen-dependent DNA and protein synthesis.