
1. Admon
2. Bay E 9736
3. Bayvit, Nimodipino
4. Brainal
5. Calnit
6. E 9736, Bay
7. Hexal, Nimodipin
8. Kenesil
9. Modus
10. Nimodipin Hexal
11. Nimodipin Isis
12. Nimodipin-isis
13. Nimodipino Bayvit
14. Nimotop
15. Nymalize
16. Remontal
1. 66085-59-4
2. Nimotop
3. Periplum
4. Admon
5. Nimodipino
6. Nymalize
7. Bay E 9736
8. Nimodipinum
9. Bay-e 9736
10. Nimodipinum [inn-latin]
11. Nimodipino [inn-spanish]
12. Bay-e-9736
13. Nemotan
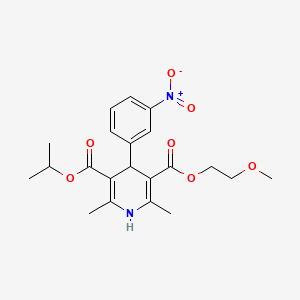
14. 3-isopropyl 5-(2-methoxyethyl) 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
15. Isopropyl 2-methoxyethyl 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate
16. Nimodipine Ap
17. Chebi:7575
18. Nimodipine (nimotop)
19. 3,5-pyridinedicarboxylic Acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, 2-methoxyethyl 1-methylethyl Ester
20. Nsc-758476
21. 57wa9qz5wh
22. Mls000863294
23. 3-o-(2-methoxyethyl) 5-o-propan-2-yl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
24. 3-(2-methoxyethyl) 5-propan-2-yl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
25. Ncgc00015714-09
26. Smr000058300
27. Dsstox_cid_3370
28. Dsstox_rid_76998
29. Isopropyl 2-methoxyethyl 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylate
30. Dsstox_gsid_23370
31. 2-methoxyethyl Propan-2-yl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
32. Nimogel
33. Drg-0139
34. Nimotop(tm)
35. 1-methylethyl 2-(methyloxy)ethyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
36. 2,6-dimethyl-4-(3'-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylic Acid 3-beta-methoxyethyl Ester 5-isopropyl Ester
37. Sr-01000075335
38. Nimotop (tn)
39. Einecs 266-127-0
40. Unii-57wa9qz5wh
41. Brn 0459792
42. Nimovent
43. Nimodipime,(s)
44. Nimodipine [usan:usp:inn:ban]
45. Nymalize (tn)
46. Cas-66085-59-4
47. Mfcd00153848
48. Eg-1961
49. Opera_id_14
50. Spectrum_001880
51. Nimodipine [mi]
52. Specplus_000716
53. Nimodipine (usp/inn)
54. Nimodipine [inn]
55. Nimodipine [jan]
56. Prestwick0_000918
57. Prestwick1_000918
58. Prestwick2_000918
59. Prestwick3_000918
60. Spectrum2_001562
61. Spectrum3_000766
62. Spectrum4_000791
63. Spectrum5_001687
64. Nimodipine [usan]
65. N-149
66. Nimodipine [vandf]
67. Nimodipine [mart.]
68. Nimodipine [usp-rs]
69. Nimodipine [who-dd]
70. (+/-)-nimodipine
71. Cbiol_001832
72. Lopac0_000891
73. Schembl34041
74. Bspbio_000796
75. Bspbio_001397
76. Bspbio_002412
77. Kbiogr_000117
78. Kbiogr_001262
79. Kbioss_000117
80. Kbioss_002408
81. Mls000069381
82. Mls001076550
83. Mls001424155
84. Mls002154061
85. Mls002172461
86. Mls003899190
87. Divk1c_006812
88. Spectrum1503600
89. Spbio_001464
90. Spbio_002985
91. Bpbio1_000876
92. Gtpl2523
93. Chembl3197349
94. Dtxsid5023370
95. Nimodipine [orange Book]
96. Schembl22882841
97. Kbio1_001756
98. Kbio2_000117
99. Kbio2_002403
100. Kbio2_002685
101. Kbio2_004971
102. Kbio2_005253
103. Kbio2_007539
104. Kbio3_000233
105. Kbio3_000234
106. Kbio3_001632
107. 3-isopropyl 5-(2-methoxyethyl)
108. Brd8407
109. Nimodipine [ep Monograph]
110. Nimodipine [usp Impurity]
111. Bio1_000118
112. Bio1_000607
113. Bio1_001096
114. Bio2_000117
115. Bio2_000597
116. Hms1361f19
117. Hms1570h18
118. Hms1791f19
119. Hms1922e04
120. Hms1989f19
121. Hms2052m05
122. Hms2089h13
123. Hms2093g11
124. Hms2097h18
125. Hms2234b05
126. Hms3262d04
127. Hms3266o22
128. Hms3369g07
129. Hms3394m05
130. Hms3402f19
131. Hms3411i12
132. Hms3657i03
133. Hms3675i12
134. Hms3714h18
135. Nimodipine [usp Monograph]
136. Pharmakon1600-01503600
137. 3,5-pyridinecarboxylic Acid 2-methoxyethyl 1-methylethyl Ester
138. Act02628
139. Albb-015317
140. Amy40399
141. Bcp21152
142. Brd-8407
143. Hy-b0265
144. 2,6-dimethyl-4-(3-nitrophenyl)-
145. Tox21_110202
146. Tox21_500891
147. Bbl028286
148. Bdbm50101971
149. Ca-211
150. Ccg-39340
151. Nsc758476
152. S1747
153. Stk642934
154. Akos005174934
155. Akos015852459
156. Nimodipine - Cas 66085-59-4
157. Nimodipine 100 Microg/ml In Methanol
158. Tox21_110202_1
159. Ac-8484
160. Ccg-101085
161. Db00393
162. Ks-1304
163. Lp00891
164. Nc00335
165. Nsc 758476
166. Sdccgsbi-0050866.p004
167. Idi1_033867
168. Isopropyl 2-methoxyethyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
169. Ncgc00015714-04
170. Ncgc00015714-05
171. Ncgc00015714-06
172. Ncgc00015714-07
173. Ncgc00015714-08
174. Ncgc00015714-10
175. Ncgc00015714-11
176. Ncgc00015714-12
177. Ncgc00015714-13
178. Ncgc00015714-16
179. Ncgc00015714-28
180. Ncgc00021456-02
181. Ncgc00024675-02
182. Ncgc00024675-03
183. Ncgc00024675-04
184. Ncgc00024675-05
185. Ncgc00024675-06
186. Ncgc00024675-07
187. Ncgc00024675-08
188. Ncgc00024675-09
189. Ncgc00261576-01
190. Nimodipine 100 Microg/ml In Acetonitrile
191. O5-isopropyl O3-(2-methoxyethyl) 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
192. Smr002530605
193. Sbi-0050866.p003
194. Ab00513963
195. Eu-0100891
196. Ft-0602600
197. N0896
198. Sw219238-1
199. C07267
200. D00438
201. F20554
202. 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-
203. Ab00053314-03
204. Ab00053314-04
205. Ab00053314_05
206. Ab00053314_06
207. 085n594
208. Q421429
209. Sr-01000075335-1
210. Sr-01000075335-2
211. Sr-01000075335-4
212. Sr-01000075335-5
213. Sr-01000075335-7
214. Brd-a58048407-001-06-3
215. Brd-a58048407-001-09-7
216. Bay E 9736 Pound>> Bay-e-9736 Pound>> Baye97
217. Nimodipine, British Pharmacopoeia (bp) Reference Standard
218. Nimodipine, European Pharmacopoeia (ep) Reference Standard
219. Nimodipine, United States Pharmacopeia (usp) Reference Standard
220. Nimodipine, Pharmaceutical Secondary Standard; Certified Reference Material
221. Nimodipine For Peak Identification, Europepharmacopoeia (ep) Reference Standard
222. 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic Acid 2-methyloxyethyl 1-methylethyl Ester
223. 2-methoxyethyl 1-methylethyl 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylate
224. 3,5-pyridinedicarboxylic Acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, 3-(2-methoxyethyl) 5-(1-methylethyl) Ester
225. 3,5-pyridinedicarboxylic Acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-,?2-methoxyethyl 1-methylethyl Ester
226. 3-isopropyl 5-(2-methoxyethyl) 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydro-3,5-pyridinedicarboxylate #
227. 3-isopropyl5-(2-methoxyethyl)2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
228. Isopropyl 2-methoxyethyl 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)pyridine-3,5-dicarboxylate
229. O5-(2-methoxyethyl) O3-propan-2-yl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
| Molecular Weight | 418.4 g/mol |
|---|---|
| Molecular Formula | C21H26N2O7 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 9 |
| Exact Mass | 418.17400117 g/mol |
| Monoisotopic Mass | 418.17400117 g/mol |
| Topological Polar Surface Area | 120 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 736 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Nimodipine |
| PubMed Health | Nimodipine (By mouth) |
| Drug Classes | Cardiovascular Agent |
| Drug Label | Nimodipine belongs to the class of pharmacological agents known as calcium channel blockers. Nimodipine is isopropyl 2 - methoxyethyl 1, 4 - dihydro - 2, 6 - dimethyl - 4 - (m-nitrophenyl) - 3, 5 pyridinedicarboxylate.It has a molecular weight... |
| Active Ingredient | Nimodipine |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 30mg |
| Market Status | Prescription |
| Company | Thepharmanetwork; Sun Pharm Inds; Barr Labs; Banner Pharmacaps |
| 2 of 4 | |
|---|---|
| Drug Name | Nymalize |
| PubMed Health | Nimodipine (By mouth) |
| Drug Classes | Cardiovascular Agent |
| Drug Label | NYMALIZE contains nimodipine, a dihydropyridine calcium channel blocker. Nimodipine is isopropyl 2-methoxyethyl 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate. It has a molecular weight of 418.5 and a molecular formula of C21H26... |
| Active Ingredient | Nimodipine |
| Dosage Form | Solution |
| Route | Oral |
| Strength | 60mg/20ml |
| Market Status | Prescription |
| Company | Arbor Pharms |
| 3 of 4 | |
|---|---|
| Drug Name | Nimodipine |
| PubMed Health | Nimodipine (By mouth) |
| Drug Classes | Cardiovascular Agent |
| Drug Label | Nimodipine belongs to the class of pharmacological agents known as calcium channel blockers. Nimodipine is isopropyl 2 - methoxyethyl 1, 4 - dihydro - 2, 6 - dimethyl - 4 - (m-nitrophenyl) - 3, 5 pyridinedicarboxylate.It has a molecular weight... |
| Active Ingredient | Nimodipine |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 30mg |
| Market Status | Prescription |
| Company | Thepharmanetwork; Sun Pharm Inds; Barr Labs; Banner Pharmacaps |
| 4 of 4 | |
|---|---|
| Drug Name | Nymalize |
| PubMed Health | Nimodipine (By mouth) |
| Drug Classes | Cardiovascular Agent |
| Drug Label | NYMALIZE contains nimodipine, a dihydropyridine calcium channel blocker. Nimodipine is isopropyl 2-methoxyethyl 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate. It has a molecular weight of 418.5 and a molecular formula of C21H26... |
| Active Ingredient | Nimodipine |
| Dosage Form | Solution |
| Route | Oral |
| Strength | 60mg/20ml |
| Market Status | Prescription |
| Company | Arbor Pharms |
For use as an adjunct to improve neurologic outcome following subarachnoid hemorrhage (SAH) from ruptured intracranial berry aneurysms by reducing the incidence and severity of ischemic deficits.
FDA Label
Treatment of aneurysmal subarchnoidal haemorrhage
Nimodipine belongs to the class of pharmacological agents known as calcium channel blockers. Nimodipine is indicated for the improvement of neurological outcome by reducing the incidence and severity of ischemic deficits in patients with subarachnoid hemorrhage from ruptured congenital aneurysms who are in good neurological condition post-ictus (e.g., Hunt and Hess Grades I-III). The contractile processes of smooth muscle cells are dependent upon calcium ions, which enter these cells during depolarization as slow ionic transmembrane currents. Nimodipine inhibits calcium ion transfer into these cells and thus inhibits contractions of vascular smooth muscle. In animal experiments, nimodipine had a greater effect on cerebral arteries than on arteries elsewhere in the body perhaps because it is highly lipophilic, allowing it to cross the blood brain barrier.
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Calcium Channel Blockers
A class of drugs that act by selective inhibition of calcium influx through cellular membranes. (See all compounds classified as Calcium Channel Blockers.)
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
C08CA06
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C08 - Calcium channel blockers
C08C - Selective calcium channel blockers with mainly vascular effects
C08CA - Dihydropyridine derivatives
C08CA06 - Nimodipine
Absorption
In humans, nimodipine is rapidly absorbed after oral administration, and peak concentrations are generally attained within one hour. Bioavailability is 100% following intravenous administration and 3-30% following oral administration due to extensive first-pass metabolism.
Route of Elimination
Nimodipine is eliminated almost exclusively in the form of metabolites and less than 1% is recovered in the urine as unchanged drug. Numerous metabolites, all of which are either inactive or considerably less active than the parent compound, have been identified.
Hepatic metabolism via CYP 3A4.
Nimodipine has known human metabolites that include 2,6-Dimethyl-4-(3-nitrophenyl)-5-propan-2-yloxycarbonyl-1,4-dihydropyridine-3-carboxylic acid, Dehydro nimodipine, and Unii-96S4GG1upr.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
1.7-9 hours
Although the precise mechanism of action is not known, nimodipine blocks intracellular influx of calcium through voltage-dependent and receptor-operated slow calcium channels across the membranes of myocardial, vascular smooth muscle, and neuronal cells. By specifically binding to L-type voltage-gated calcium channels, nimodipine inhibits the calcium ion transfer, resulting in the inhibition of vascular smooth muscle contraction. Evidence suggests that the dilation of small cerebral resistance vessels, with a resultant increase in collateral circulation, and/or a direct effect involving the prevention of calcium overload in neurons may be responsible for nimodipine's clinical effect in patients with subarachnoid hemorrhage.