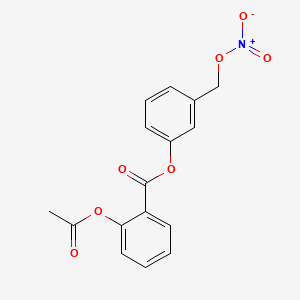

1. 2-acetoxybenzoate-2-(1-nitroxymethyl)phenyl Ester
2. Ncx 4016
3. Ncx-4016
4. Nitric Oxide-releasing Aspirin
1. 175033-36-0
2. Ncx-4016
3. No-aspirin 1
4. Ncx 4016
5. [3-(nitrooxymethyl)phenyl] 2-acetyloxybenzoate
6. No-asa
7. 3-((nitrooxy)methyl)phenyl 2-(acetyloxy)benzoate
8. Eh04h13l6b
9. Benzoic Acid, 2-(acetyloxy)-, 3-((nitrooxy)methyl)phenyl Ester
10. 3-[(nitrooxy)methyl]phenyl 2-(acetyloxy)benzoate
11. M-no-asa
12. Benzoic Acid, 2-(acetyloxy)-, 3-[(nitrooxy)methyl]phenyl Ester
13. 3-(nitroxymethyl)phenyl 2-acetoxybenzoate
14. M-no-aspirin
15. Nitric Oxide-releasing Aspirin
16. 2-acetoxybenzoate-2-(1-nitroxymethyl)phenyl Ester
17. 2-((nitrooxy)methyl)phenyl 2-(acetyloxy)benzoate
18. 2-acetoxybenzoic Acid 3-nitrooxymethylphenyl Ester
19. Unii-eh04h13l6b
20. Benzoic Acid, 2-(acetyloxy)-, 2-((nitrooxy)methyl)phenyl Ester
21. Schembl19524
22. Chembl374385
23. Gtpl9018
24. Zinc22315
25. Dtxsid60938618
26. Ncx4016
27. Chebi:125482
28. Bcp24321
29. Benzoic Acid,2-(acetyloxy)-, 3-[(nitrooxy)methyl]phenyl Ester
30. Akos025294730
31. Ccg-208118
32. Db12445
33. Hy-123823
34. 3-((nitrooxy)methyl)phenyl2-acetoxybenzoate
35. Cs-0086219
36. Ft-0673020
37. Ncx 4016, >=98% (hplc)
38. 3-((nitrooxy)methyl)phenyl 2-acetoxybenzoate
39. 2-acetoxy Benzoic Acid-3-nitrooxymethyl Phenyl Ester
40. J-011058
41. Q3877377
42. Brd-k08807999-001-01-6
43. 2-(acetyloxy)-(3-(nitroxymethyl)phenyl)benzoic Acid
44. (2-{[(1s)-1-(5-fluoro(2-pyridyl))ethyl]amino}-5-chloropyrimidin-4-yl)[5-(methylethoxy)pyrazol-3-yl]amine
| Molecular Weight | 331.28 g/mol |
|---|---|
| Molecular Formula | C16H13NO7 |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 7 |
| Exact Mass | 331.06920175 g/mol |
| Monoisotopic Mass | 331.06920175 g/mol |
| Topological Polar Surface Area | 108 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 462 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)
Cysteine Proteinase Inhibitors
Exogenous and endogenous compounds which inhibit CYSTEINE ENDOPEPTIDASES. (See all compounds classified as Cysteine Proteinase Inhibitors.)
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Fibrinolytic Agents
Fibrinolysin or agents that convert plasminogen to FIBRINOLYSIN. (See all compounds classified as Fibrinolytic Agents.)