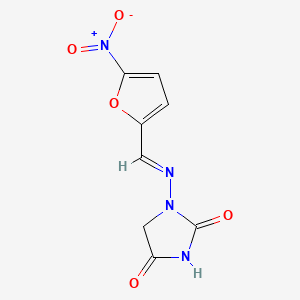

1. Furadantin
2. Furadantine
3. Furadoine
4. Furadonine
5. Furantoin
6. Macrodantin
7. Nitrofurantoin Sodium Salt
8. Nitrofurantoin, Monohydrate
1. 67-20-9
2. Macrodantin
3. Furadantin
4. 5-nitrofurantoin
5. Furadonine
6. Furadantine
7. Furadantoin
8. Furadoine
9. Furadontin
10. Furantoin
11. Nifurantin
12. Furalan
13. Nitrofuradantin
14. Nitrofurantoine
15. Berkfurin
16. Chemiofuran
17. Furachel
18. Furadonin
19. Furatoin
20. Furobactina
21. Macrobid
22. Novofuran
23. Orafuran
24. Parfuran
25. Trantoin
26. Urantoin
27. Urofurin
28. Welfurin
29. Zoofurin
30. Cyantin
31. Cystit
32. Furina
33. Ituran
34. Nitoin
35. Urizept
36. Urodin
37. Urolong
38. Furadantine Mc
39. Uro-tablinen
40. Fur-ren
41. Macrofuran
42. Nitrofurantoina
43. Benkfuran
44. Dantafur
45. Furaloid
46. Nierofu
47. Macpac
48. Nitrex
49. Nitrofurantoinum
50. Fua Med
51. N-toin
52. Usaf Ea-2
53. 1-(5-nitro-2-furfurylideneamino)hydantoin
54. 1-((5-nitrofurfurylidene)amino)hydantoin
55. N-(5-nitrofurfurylidene)-1-aminohydantoin
56. Nitrofurantoin, Macrocrystalline
57. 1-[(5-nitrofurfurylidene)amino]hydantoin
58. 1-(5-nitro-2-furfurylidenamino)hydantoin
59. N-(5-nitro-2-furfurylidene)-1-aminohydantoin
60. Nitrofurantoin Anhydrous
61. Nci-c55196
62. Nsc 2107
63. Nitrofurantoin Macrocrystalline
64. Nsc 44150
65. N-(5-nitro-2-furfurylideno)-1-aminohydantoina
66. Nsc-2107
67. Furadoninum
68. Nitrofurantoin Macrocrystal
69. 1-(((5-nitrofuran-2-yl)methylene)amino)imidazolidine-2,4-dione
70. 2,4-imidazolidinedione, 1-[[(5-nitro-2-furanyl)methylene]amino]-
71. 1-[(e)-(5-nitrofuran-2-yl)methylideneamino]imidazolidine-2,4-dione
72. Nitrofurantoin, Macrocrystals
73. Mls000028500
74. Cistofuran
75. Macrodantina
76. Macrofurin
77. Nifuretten
78. Alfuran
79. Berkfuran
80. Ceduran
81. Furabid
82. Furedan
83. Gerofuran
84. Phenurin
85. Siraliden
86. Uerineks
87. Urofuran
88. Urolisa
89. Chebi:71415
90. Hydantoin, 1-((5-nitrofurfurylidene)amino)-
91. Piyeloseptyl
92. 1-[[(5-nitro-2-furanyl)methylene]amino]-2,4-imidazolidinedione
93. Furadantina Mc
94. Furadantine-mc
95. 2,4-imidazolidinedione, 1-(((5-nitro-2-furanyl)methylene)amino)-
96. Furophen T
97. Nitrofur-c
98. Ro-antoin
99. Furadantin Retard
100. Uro-selz
101. Ivadantin
102. 927ah8112l
103. Smr000058271
104. 1-(((5-nitrofuran-2-yl)methylene)-amino)imidazolidine-2,4-dione
105. 1-{[(1e)-(5-nitrofuran-2-yl)methylidene]amino}imidazolidine-2,4-dione
106. Nitrofurantoina [dcit]
107. Nitrofurantoine [inn-french]
108. Nitrofurantoinum [inn-latin]
109. Furodantin
110. 1-((5-nitro-2-furanyl)methylene)amino-2,4-imidazolidenedione
111. Fuamed
112. 1-{[(e)-(5-nitrofuran-2-yl)methylidene]amino}imidazolidine-2,4-dione
113. Nsc-44150
114. Furadantin (tn)
115. Macrobid (tn)
116. 178170-37-1
117. Ccris 1192
118. Hydantoin, 1-[(5-nitrofurfurylidene)amino]-
119. Hsdb 3135
120. Sr-05000001681
121. Einecs 200-646-5
122. Nsc2107
123. Furantoina
124. Furadoin
125. Uvamin
126. N-(5-nitro-2-furfurylideno)-1-aminohydantoina [polish]
127. Nsc44150
128. Ai3-26388
129. (e)-1-(((5-nitrofuran-2-yl)methylene)amino)imidazolidine-2,4-dione
130. Nitrofurantoin (jan/usp/inn)
131. Unii-927ah8112l
132. Furophen T-caps
133. Ncgc00091505-01
134. Ncgc00091505-07
135. Prestwick_358
136. Nitrofurantoin Macro
137. 5-nitrofurantoindorn
138. J01xe01
139. Nitrofurantoin [usp:inn:ban:jan]
140. Mfcd00003224
141. Nd-3320
142. Nd-7248
143. Nitrofurantoin, 97%
144. Nitrofurantoinum Anhydrous
145. Prestwick2_000168
146. Prestwick3_000168
147. Spectrum5_001367
148. Nitrofurantoin, Crystalline
149. Chembl572
150. Nitrofurantoin [mi]
151. Nitrofurantoin Macrocrystals
152. Cid_4509
153. Nitrofurantoin [inn]
154. Nitrofurantoin [jan]
155. Schembl29470
156. Schembl29472
157. Bspbio_000035
158. Bspbio_002073
159. Nitrofurantoin [hsdb]
160. Nitrofurantoin [iarc]
161. Bidd:gt0181
162. Nitrofurantoin [vandf]
163. Spectrum1500433
164. Nitrofurantoin [mart.]
165. Bpbio1_000039
166. Nitrofurantoin [usp-rs]
167. Nitrofurantoin [who-dd]
168. Bdbm57045
169. Chebi:95222
170. Hms500l06
171. Hms1568b17
172. Hms1920p21
173. Hms2091h16
174. Hms2095b17
175. Hms3712b17
176. Pharmakon1600-01500433
177. Nitrofurantoin [green Book]
178. 1-[(e)-(5-nitro-2-furyl)methyleneamino]imidazolidine-2,4-dione
179. Hy-a0090
180. Zinc7997568
181. Nitrofurantoin [orange Book]
182. 2,4-imidazolidenedione, 1-(((5-nitro-2-furanyl)methylene)amino)-
183. Ccg-40108
184. Nitrofurantoin [ep Monograph]
185. Nsc757243
186. S4536
187. Stk009471
188. Stl454163
189. Nitrofurantoin [usp Monograph]
190. Akos001678301
191. Db00698
192. Nsc-757243
193. Idi1_000224
194. Macrobid Component Nitrofurantoin
195. Ncgc00091505-03
196. Ncgc00091505-04
197. Ncgc00091505-05
198. Ncgc00091505-06
199. Ncgc00091505-08
200. Ncgc00091505-09
201. Ncgc00091505-10
202. Nitrofurantoin Anhydrous [who-ip]
203. Ls-13402
204. 1-((5-nitrofurfurylidene)amino)-hydantoin
205. Nitrofurantoin Component Of Macrobid
206. Sbi-0051457.p003
207. Ab00513815
208. Bb 0310231
209. N0883
210. Nitrofurantoin Macrocrystalline [vandf]
211. A16008
212. C07268
213. D00439
214. Ab00052052_03
215. Nitrofurantoinum Anhydrous [who-ip Latin]
216. A835659
217. Hydantoin, N-(5-nitro-2-furfurylidene)-1-amino-
218. Nitrofurantoin, Vetranal(tm), Analytical Standard
219. Q-201479
220. Sr-05000001681-1
221. Sr-05000001681-2
222. Sr-05000001681-3
223. Sr-05000001681-4
224. Brd-k76927775-001-05-0
225. Nitrofurantoin, Antibiotic For Culture Media Use Only
226. Hydantoin, 1-[(5-nitrofurfurylidene)amino]- (7ci,8ci)
227. Macrobid Component Nitrofurantoin, Macrocrystalline
228. 1-{[(5-nitro-2-furyl)methylene]amino}imidazolidine-2,4-dione
229. Nitrofurantoin, Macrocrystalline Component Of Macrobid
230. (e)-1-[(5-nitro-2-furyl)methylideneamino]imidazolidine-2,4-dione
231. 1-([(5-nitro-2-furyl)methylidene]amino)-2,4-imidazolidinedione #
232. 1-[(e)-(5-nitro-2-furanyl)methylideneamino]imidazolidine-2,4-dione
233. 1-{[(5-nitrofuran-2-yl)methylidene]amino}imidazolidine-2,4-dione
234. Nitrofurantoin, United States Pharmacopeia (usp) Reference Standard
235. 1-{[(e)-1-(5-nitro-2-furyl)methylidene]amino}-1h-imidazole-2,4(3h,5h)-dione
236. 4-hydroxy-1-{[(e)-(5-nitrofuran-2-yl)methylidene]amino}-1,5-dihydro-2h-imidazol-2-one
237. N-(5-nitro-2-furfurylidine)-1-aminohydantoin; N-(5-nitrofurfurylidene)-1aminohydantoin
238. Nitrofurantoin, Pharmaceutical Secondary Standard; Certified Reference Material
1. Nitrofurantoin [monohydrate]
2. 1-((5-nitrofurfurylidene)amino)hydantoin Monohydrate
3. Anw-71970
4. Nifurantin Monohydrate
| Molecular Weight | 238.16 g/mol |
|---|---|
| Molecular Formula | C8H6N4O5 |
| XLogP3 | -0.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 238.03381930 g/mol |
| Monoisotopic Mass | 238.03381930 g/mol |
| Topological Polar Surface Area | 121 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 390 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 8 | |
|---|---|
| Drug Name | Furadantin |
| PubMed Health | Nitrofurantoin |
| Drug Classes | Antibiotic |
| Drug Label | Furadantin (nitrofurantoin), a synthetic chemical, is a stable, yellow, crystalline compound. Furadantin is an antibacterial agent for specific urinary tract infections. Furadantin is available in 25mg/5mL liquid suspension for oral administration.1-... |
| Active Ingredient | Nitrofurantoin |
| Dosage Form | Suspension |
| Route | Oral |
| Strength | 25mg/5ml |
| Market Status | Prescription |
| Company | Shionogi |
| 2 of 8 | |
|---|---|
| Drug Name | Macrobid |
| PubMed Health | Nitrofurantoin Combination (By mouth) |
| Drug Classes | Antibiotic |
| Active Ingredient | nitrofurantoin, macrocrystalline; Nitrofurantoin |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 25mg; 75mg |
| Market Status | Prescription |
| Company | Alvogen |
| 3 of 8 | |
|---|---|
| Drug Name | Macrodantin |
| PubMed Health | Nitrofurantoin |
| Drug Classes | Antibiotic |
| Drug Label | Macrodantin (nitrofurantoin macrocrystals) is a synthetic chemical of controlled crystal size. It is a stable, yellow, crystalline compound. Macrodantin is an antibacterial agent for specific urinary tract infections. It is available in 25 mg, 50 mg,... |
| Active Ingredient | Nitrofurantoin, macrocrystalline |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 25mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Alvogen |
| 4 of 8 | |
|---|---|
| Drug Name | Nitrofurantoin |
| PubMed Health | Nitrofurantoin |
| Drug Classes | Antibiotic |
| Drug Label | Nitrofurantoin oral suspension, USP, a synthetic chemical, is a stable, yellow, crystalline compound. Nitrofurantoin oral suspension, USP is an antibacterial agent for specific urinary tract infections. Nitrofurantoinoral suspension, USP is available... |
| Active Ingredient | Nitrofurantoin, macrocrystalline; Nitrofurantoin |
| Dosage Form | Capsule; Suspension |
| Route | Oral |
| Strength | 25mg/5ml; 100mg; 50mg |
| Market Status | Prescription |
| Company | Amneal Pharms; Novel Labs; Ivax Sub Teva Pharms; Caraco; Mylan |
| 5 of 8 | |
|---|---|
| Drug Name | Furadantin |
| PubMed Health | Nitrofurantoin |
| Drug Classes | Antibiotic |
| Drug Label | Furadantin (nitrofurantoin), a synthetic chemical, is a stable, yellow, crystalline compound. Furadantin is an antibacterial agent for specific urinary tract infections. Furadantin is available in 25mg/5mL liquid suspension for oral administration.1-... |
| Active Ingredient | Nitrofurantoin |
| Dosage Form | Suspension |
| Route | Oral |
| Strength | 25mg/5ml |
| Market Status | Prescription |
| Company | Shionogi |
| 6 of 8 | |
|---|---|
| Drug Name | Macrobid |
| PubMed Health | Nitrofurantoin Combination (By mouth) |
| Drug Classes | Antibiotic |
| Active Ingredient | nitrofurantoin, macrocrystalline; Nitrofurantoin |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 25mg; 75mg |
| Market Status | Prescription |
| Company | Alvogen |
| 7 of 8 | |
|---|---|
| Drug Name | Macrodantin |
| PubMed Health | Nitrofurantoin |
| Drug Classes | Antibiotic |
| Drug Label | Macrodantin (nitrofurantoin macrocrystals) is a synthetic chemical of controlled crystal size. It is a stable, yellow, crystalline compound. Macrodantin is an antibacterial agent for specific urinary tract infections. It is available in 25 mg, 50 mg,... |
| Active Ingredient | Nitrofurantoin, macrocrystalline |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 25mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Alvogen |
| 8 of 8 | |
|---|---|
| Drug Name | Nitrofurantoin |
| PubMed Health | Nitrofurantoin |
| Drug Classes | Antibiotic |
| Drug Label | Nitrofurantoin oral suspension, USP, a synthetic chemical, is a stable, yellow, crystalline compound. Nitrofurantoin oral suspension, USP is an antibacterial agent for specific urinary tract infections. Nitrofurantoinoral suspension, USP is available... |
| Active Ingredient | Nitrofurantoin, macrocrystalline; Nitrofurantoin |
| Dosage Form | Capsule; Suspension |
| Route | Oral |
| Strength | 25mg/5ml; 100mg; 50mg |
| Market Status | Prescription |
| Company | Amneal Pharms; Novel Labs; Ivax Sub Teva Pharms; Caraco; Mylan |
Anti-Infective Agents, Urinary /SRP: Antibacterial/
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
NITROFURANTOIN IS BACTERIOSTATIC AT CONCN OF 5-10 UG/ML & BACTERICIDAL AT 100 UG/ML, BUT IT IS NOT KNOWN WHETHER BACTERICIDAL ACTION OCCURS IN VIVO. ANTIBACTERIAL ACTIVITY IS HIGHER IN ACIDIC URINE. ...SUPERSATURATED SOLN OF NITROFURANTOIN DO NOT CAUSE CRYSTALLURIA.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1008
NITROFURANTOIN IS ACTIVE AGAINST MANY STRAINS OF COMMON URINARY TRACT PATHOGENS E COLI, PROTEUS SPECIES, PSEUDOMONAS...ENTEROBACTER, AND STAPHYLOCOCCI, AS WELL AS ENTEROCOCCI, STREPTOCOCCI, CLOSTRIDIA, & BACILLUS SUBTILIS.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1008
...APPROVED ONLY FOR TREATMENT OF URINARY TRACT INFECTIONS CAUSED BY MICROORGANISMS THAT ARE KNOWN TO BE SENSITIVE TO DRUG. ... IT HAS BEEN USED EFFECTIVELY TO PREVENT RECURRENT INFECTIONS & FOR PREVENTION OF BACTERIURIA AFTER PROSTATECTOMY.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1070
For more Therapeutic Uses (Complete) data for NITROFURANTOIN (8 total), please visit the HSDB record page.
A COURSE OF THERAPY SHOULD NOT EXCEED 14 DAYS, & REPEATED COURSES SHOULD BE SEPARATED BY REST PERIODS. ... PREGNANT WOMEN AT TERM, INDIVIDUALS WITH IMPAIRED RENAL FUNCTION (CREATININE CLEARANCE LESS THAN 40 ML/MIN), & CHILDREN BELOW 1 MONTH OF AGE SHOULD NOT RECEIVE NITROFURANTOIN.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1070
Maternal Medication Usually Compatible with Breast-Feeding: Nitrofurantoin: Hemolysis in infant with glucose-6-phosphate dehydrogenase (G-6-PD) deficiency. /from Table 6/
Report of the American Academy of Pediatrics Committee on Drugs in Pediatrics 93 (1): 141 (1994)
...MOST SPECIES OF PROTEUS & PSEUDOMONAS & MANY OF ENTEROBACTER & KLEBSIELLA ARE RESISTANT. ANTIBACTERIAL CONCN ARE NOT ACHIEVED IN PLASMA FOLLOWING INGESTION OF RECOMMENDED DOSES, BECAUSE DRUG IS RAPIDLY ELIMINATED. ...IN PT WITH IMPAIRED GLOMERULAR FUNCTION EFFICACY OF DRUG MAY BE DECR & SYSTEMIC TOXICITY INCR.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1069
INJECTION OF NITROFURANTOIN SODIUM IS INDICATED ONLY FOR USE IN ACUTELY ILL PT WHO CANNOT TOLERATE ORAL NITROFURANTOIN. /SODIUM NITROFURANTOIN/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1009
For more Drug Warnings (Complete) data for NITROFURANTOIN (31 total), please visit the HSDB record page.
Nitrofurantoin is indicated to treat acute uncomplicated urinary tract infections.
FDA Label
Nitrofurantoin interferes with vital processes in bacteria, which leads to their death. Nitrofurantoin rapidly reaches therapeutic concentrations in the urine and is also cleared rapidly.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Anti-Infective Agents, Urinary
Substances capable of killing agents causing urinary tract infections or of preventing them from spreading. (See all compounds classified as Anti-Infective Agents, Urinary.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01X - Other antibacterials
J01XE - Nitrofuran derivatives
J01XE01 - Nitrofurantoin
Absorption
Nitrofurantoin reaches a Cmax of 0.875-0.963mg/L with an AUC of 2.21-2.42mg\*h/L. It is 38.8-44.3% bioavailable. Taking nitrofurantoin with food increases the absorption and duration of therapeutic concentrations in the urine.
Route of Elimination
27-50% of an oral dose is excreted in the urine as unchanged nitrofurantoin. 90% of the total dose is eliminated in the urine.
Volume of Distribution
Data regarding the volume of distribution in humans is scarce but it has been reported as 0.46L/kg in dogs.
Clearance
The clearance of nitrofurantoin is 16.7-19.4L/h.
.../IT/ IS RAPIDLY & COMPLETELY ABSORBED FROM GI TRACT. ... PLASMA HALF-LIFE IS 0.3 TO 1 HR; ABOUT 40% IS EXCRETED UNCHANGED INTO URINE. AVG DOSE OF NITROFURANTOIN YIELDS URINE CONCN OF APPROX 200 UG/ML. ... RATE OF EXCRETION IS LINEARLY RELATED TO CREATININE CLEARANCE...
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1069
CLINICAL STUDIES...INDICATE THAT IN NORMAL FASTING INDIVIDUALS, LESS NITROFURANTOIN IS ABSORBED & AT SLOWER RATE FROM MACROCRYSTALLINE THAN MICROCRYSTALLINE FORM. PRESENCE OF FOOD IN INTESTINE DELAYS ABSORPTION OF BOTH FORMS APPRECIABLY, INCR PEAK LEVELS OF MACROCRYSTALLINE COMPD, BUT NOT MICROCRYSTALLINE COMPD, ENHANCES BIOAVAILABILITY OF BOTH FORMS, & PROLONGS DURATION OF THERAPEUTIC URINARY CONCN.
American Medical Association, AMA Department of Drugs. AMA Drug Evaluations. 4th ed. Chicago: American Medical Association, 1980., p. 1323
ENHANCEMENT OF...ABSORPTION BY FOOD RANGED FROM 20 TO 400%, WITH GREATEST EFFECT OCCURRING WITH LEAST SOLUBLE DOSAGE FORMS. .../IT/ IS INEFFICIENTLY ABSORBED FROM RECTAL SUPPOSITORIES...
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 5: A Review of the Literature Published during 1976 and 1977. London: The Chemical Society, 1979., p. 76
NITROFURANTOIN ABSORPTION IS SIGNIFICANTLY INCR IN MAN FROM A DRUG-DEOXYCHOLIC ACID CO-PRECIPITATE COMPARED WITH PHYS MIXT, & FASTER ABSORPTION...FROM CO-PRECIPITATE WAS ASSOCIATED WITH FASTER IN VITRO DISSOLUTION RATE. ... EXCRETED IN BILE OF DOGS & ABOUT 1/3 OF THAT EXCRETED IS REABSORBED FROM INTESTINE WITHIN 3 HR.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 187
For more Absorption, Distribution and Excretion (Complete) data for NITROFURANTOIN (13 total), please visit the HSDB record page.
0.8-1.8% of a dose is metabolized to aminofurantoin, and 0.9% of a dose is metabolized to other metabolites.
AFTER DOSE OF 0.200 MG/KG, 22% IS EXCRETED IN URINE AS N-(5-NITROFURFURYLIDENEAMINO)-2-IMIDAZOLINE-ONE. /FROM TABLE/
Sunshine, I. (ed.). CRC Handbook of Analytical Toxicology. Cleveland: The Chemical Rubber Co., 1969., p. 356
READILY DEGRADED BY ALL /BODY/ TISSUES (EXCEPT BLOOD) INTO INACTIVE METABOLITES-HYDROXYLAMINO COMPD & AMINOFURALDEHYDENITROFURIC ACID. /HUMAN, ORAL/
American Society of Hospital Pharmacists. Data supplied on contract from American Hospital Formulary Service and other current ASHP sources., p. 1971
AFTER NITROFURANTOIN (50 MG) IV INFUSION, 47% OF THE DOSE WAS EXCRETED UNCHANGED IN THE URINE AND 1.2% WAS RECOVERED AS THE REDUCED METABOLITE AMINOFURANTOIN.
HOENER BA, PATTERSON SE; NITROFURANTOIN DISPOSITION; CLIN PHARMACOL THER 29(6) 808 (1981)
Nitrofurantoin is partially metabolized, mainly in the liver. A small fraction of the drug is reduced to form aminofurantoin.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 97. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1997 (Plus Supplements)., p. 632
The half life of nitrofurantoin is 0.72-0.78h.
PLASMA HALF-LIFE IS 0.3 TO 1 HR...
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1069
NITROFURANTOIN HALF-LIFE WAS 0.41 HOURS IN ADULTS AND 0.95 HOURS IN 2-WEEK-OLD RATS.
WIERZBA K ET AL; PAEDIATR PAEDOL 17 (2): 293 (1982)
Nitrofurantoin is converted by bacterial nitroreductases to electrophilic intermediates which inhibit the citric acid cycle as well as synthesis of DNA, RNA, and protein.
MICROSOMAL AND SOLUBLE FRACTIONS FROM BOTH RAT LIVER AND LUNG MEDIATED THE COVALENT BINDING OF (14)C-LABELED NITROFURANTOIN (I) TO TISSUE MACROMOLECULES IN VITRO. OXYGEN STRONGLY INHIBITED THE BINDING IN BOTH FRACTIONS, AND CARBON MONOXIDE FAILED TO INHIBIT THE BINDING IN MICROSOMAL PREPARATIONS, INDICATING ACTIVATION OF I IN BOTH SYSTEMS BY NITROREDUCTION RATHER THAN OXIDATION OF THE FURAN RING. MICROSOMAL NITROREDUCTION AND COVALENT BINDING OF I WERE INHIBITED BY AN ANTIBODY AGAINST NADPH-CYTOCHROME C REDUCTASE AND COVALENT BINDING WAS ENHANCED BY THE ADDITION OF FAD. IN SOLUBLE FRACTIONS, MAXIMUM RATES OF COVALENT BINDING WERE OBTAINED IN THE PRESENCE OF NADH AND HYPOXANTHINE, AND IT WAS INHIBITED BY ALLOPURINOL, A XANTHINE OXIDASE INHIBITOR. REDUCED GLUTATHIONE DECREASED COVALENT BINDING OF I IN BOTH MICROSOMAL AND SOLUBLE FRACTIONS OF LIVER AND LUNG, BUT THE RATE OF NITROREDUCTION WAS UNAFFECTED.
PMID:36083 BOYD MR ET AL; BIOCHEM PHARMACOL 28 (5): 601 (1979)
THE HYPOTHESIS IS PRESENTED THAT THE TOXICITY OF NITROFURANS SUCH AS NITROFURANTOIN (I), WHICH ARE USED IN COMMERCIAL POULTRY PRODUCTION, IS DUE TO OXIDATIVE METABOLIC STRESS CAUSED BY THE O2- FREE RADICAL FORMED DURING METABOLISM OF THE COMPOUNDS.
COMBS GF JR; PROC-CORNELL NUTR CONF FEED MANUF: 9 (1979)