

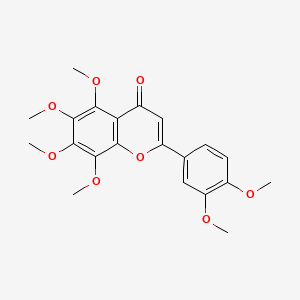

1. Hexamethoxyflavone
1. 478-01-3
2. Hexamethoxyflavone
3. 3',4',5,6,7,8-hexamethoxyflavone
4. 5,6,7,8,3',4'-hexamethoxyflavone
5. 2-(3,4-dimethoxyphenyl)-5,6,7,8-tetramethoxychromen-4-one
6. 2-(3,4-dimethoxyphenyl)-5,6,7,8-tetramethoxy-4h-1-benzopyran-4-one
7. Nsc-76751
8. D65ilj7wly
9. 2-(3,4-dimethoxyphenyl)-5,6,7,8-tetramethoxy-4h-chromen-4-one
10. 4h-1-benzopyran-4-one, 2-(3,4-dimethoxyphenyl)-5,6,7,8-tetramethoxy-
11. Chembl76447
12. Nobiletin (hexamethoxyflavone)
13. Chebi:7602
14. Nsc76751
15. Nsc-618903
16. Flavone, 5,6,7,8,3',4'-hexamethoxy
17. Smr000156231
18. Ccris 9012
19. Unii-d65ilj7wly
20. Nsc 76751
21. Mfcd03273560
22. Cpd000156231
23. Nobiletin, >=97%
24. Nobiletin [inci]
25. Spectrum2_001697
26. Spectrum3_000921
27. Spectrum4_001020
28. Nobiletin [usp-rs]
29. Kbiogr_001519
30. Mls000574877
31. Mls000759462
32. Mls000877030
33. Mls001424129
34. Nobiletin, Analytical Standard
35. Schembl244029
36. Spectrum1505268
37. Spbio_001654
38. Megxp0_000930
39. Acon1_000921
40. Kbio3_001922
41. Dtxsid30197275
42. 2-(3,4-dimethoxyphenyl)-5,6,7,8-tetramethoxy-chromen-4-one
43. Hms2051d09
44. Hms2234a09
45. Hms3373c14
46. Hms3393d09
47. Hms3651g20
48. Hexamethoxyflavone [who-dd]
49. Hy-n0155
50. Zinc1531669
51. 3'4'5,6,7,8-hexamethoxyflavone
52. Bdbm50338976
53. Ccg-38781
54. Lmpk12111468
55. Nsc618903
56. Stl565829
57. Akos015965334
58. Nobiletin, 20% (technical Grade)
59. Ac-1023
60. Cs-5518
61. Nc00186
62. Sdccgmls-0066776.p001
63. Ncgc00095703-01
64. Ncgc00095703-02
65. Ncgc00095703-06
66. Ncgc00169228-01
67. 5,6,7,8,3'',4''-hexamethoxyflavone
68. As-17452
69. Nci60_041691
70. Db-050181
71. Ft-0686667
72. N0871
73. N1311
74. S2333
75. Sw197566-2
76. A827343
77. Flavone, 3',4',5,6,7,8-hexamethoxy-
78. Sr-01000712262
79. Q-100511
80. Q2402963
81. Sr-01000712262-5
82. Brd-k06753942-001-02-0
83. 2-(3,4-dimethoxy-phenyl)-5,6,7,8-tetramethoxy-chromen-4-one
84. 4,5,6,7,8,9,10,11,12,13-decahydrocyclododeca[d]oxazole
85. 4h-1-benzopyran-4-one,4-dimethoxyphenyl)-5,6,7,8-tetramethoxy-
86. 2-(3,4-dimethoxyphenyl)-5,6,7,8-tetramethoxy-4h-1-benzopyran-4-one, 9ci
87. 3 Inverted Exclamation Mark ,4 Inverted Exclamation Mark ,5,6,7,8-hexamethoxyflavone
| Molecular Weight | 402.4 g/mol |
|---|---|
| Molecular Formula | C21H22O8 |
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 7 |
| Exact Mass | 402.13146766 g/mol |
| Monoisotopic Mass | 402.13146766 g/mol |
| Topological Polar Surface Area | 81.7 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 593 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)