


1. 17-deacetylnorgestimate
2. 18,19-dinorpregn-4-en-20-yn-3-one, 13-ethyl-17-hydroxy-, Oxime, (17alpha)-
3. Levonorgestrel Oxime
4. Levonorgestrel Oxime, (3e,17alpha)-isomer
5. Levonorgestrel Oxime, (3z,17alpha)-isomer
6. Lngo
7. Progestin Norelgestromin
1. Levonorgestrel Oxime
2. Norplant 3-oxime
3. D-norgestrel 3-oxime
4. 18-methylnorethindrone Oxime
5. 17-deacetylnorgestimate
6. 53016-31-2
7. 17-deacylnorgestimate
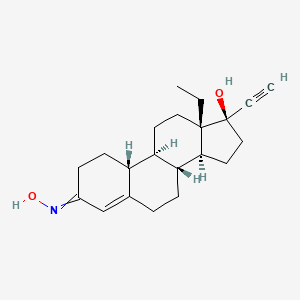
8. (8r,9s,10r,13s,14s,17r)-13-ethyl-17-ethynyl-3-hydroxyimino-1,2,6,7,8,9,10,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-ol
9. Deacetylnorgestimate
10. Norgestimate Metabolite Norelgestromin
11. Norelgestromin (17-deacetylnorgestimate)
12. Brn 4202099
13. Unii-r0tay3x631
14. Norelgestromin [usan:inn:ban]
15. Rwj 10553
16. Levonorgestrel 3-oxime
17. 17-deacetyl Norgestimate
18. 18,19-dinorpregn-4-en-20-yn-3-one, 13-ethyl-17-hydroxy-, Oxime, (17alpha)-
19. R0tay3x631
20. Chembl4791392
21. Dtxsid9046788
22. 13-ethyl-17-hydroxy-18,19-dinor-17alpha-pregn-4-en-20-yn-3-one Oxime
23. Db06713
24. 18,19-dinorpregn-4-en-20-yn-3-one, 13-ethyl-17-hydroxy-, Oxime, (8-alpha,9-beta,10-alpha,13-alpha,14-beta)-
25. 17-deacylnorgestimate17-deacetyl Norgestimate
| Molecular Weight | 327.5 g/mol |
|---|---|
| Molecular Formula | C21H29NO2 |
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 327.219829168 g/mol |
| Monoisotopic Mass | 327.219829168 g/mol |
| Topological Polar Surface Area | 52.8 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 642 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 1 |
| Covalently Bonded Unit Count | 1 |
Norelgestromin is used for contraception and menopausal hormonal therapy. Norelgestromin may potentially be used in breast cancer treatment due to its inhibitory effect on estrone sulfatase . They convert sulfated steroid precursors to estrogen during pregnancy.
Norelgestromin is used for contraception and menopausal hormonal therapy transdermally or in combination with ethinyl estradiol as a vaginal ring. Norelgestromin, in combination with ethinyl estradiol inhibits ovulation by suppressing gonadotropins.
Contraceptive Agents, Hormonal
Contraceptive agents that act on the ENDOCRINE SYSTEM. (See all compounds classified as Contraceptive Agents, Hormonal.)
G03AA13
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
Norelgestromin inhibits estrone sulfatase, which converts sulfated steroid precursors to estrogen during pregnancy. Norgelgestromin/ethinylestradiol suppresses follicular development, induces changes to the endometrium, which decreases chances of implantation and thickens the cervical mucus, impeding sperm swimming into the uterus. It also has similar agonisting binding affinities as its parent compound, Norgestimate, for progesterone and estrogen receptors.