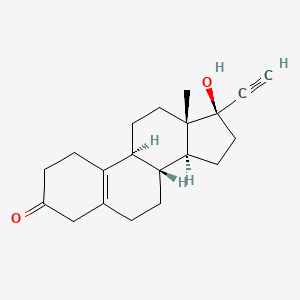

1. 19-norpregn-5(10)-en-20-yn-3-one, 17-hydroxy-, (17alpha)-
2. Norethynodrel, (8 Alpha)-(+-)-isomer
1. Noretynodrel
2. 68-23-5
3. Norethinodrel
4. Enidrel
5. Lynestrol
6. 19-norethynodrel
7. Sc-4642
8. Nsc-15432
9. 17-ethinyl-5(10)-estraeneolone
10. 19-nor-ethinyl-5,10-testosterone
11. Noretynodrel [inn]
12. Mls000069824
13. Norethinynodrel
14. Norethynodral
15. Chebi:34895
16. 88181aca0m
17. Noretynodrel (inn)
18. (17beta)-17-ethynyl-17-hydroxyestr-5(10)-en-3-one
19. (8r,9s,13s,14s,17r)-17-ethynyl-17-hydroxy-13-methyl-1,2,4,6,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-3-one
20. Norethynodrel (usan)
21. Smr000058915
22. 19-norpregn-5(10)-en-20-yn-3-one,17-hydroxy-, (17a)-
23. Norethynodrel [usan]
24. Infecundin
25. Noretinodrel [dcit]
26. Noretinodrel
27. Noretynodrelum
28. Noretynodrelum [inn-latin]
29. Ccris 486
30. 19-norpregn-5(10)-en-20-yn-3-one, 17-hydroxy-, (17.alpha.)-
31. Hsdb 2951
32. 17alpha-ethinyl-5,10-estrenolone
33. 17-alpha-ethinyl-5,10-estrenolone
34. Einecs 200-682-1
35. Nsc 15432
36. Norethynodrel [progestins]
37. Norethynodrel [usan:usp]
38. Cb 8023
39. Brn 2389771
40. 17-hydroxy-19-nor-17.alpha.-pregn-5(10)-en-20-yn-3-one
41. Nsc15432
42. Unii-88181aca0m
43. 17alpha-ethynyl-5(10)-estren-17-ol-3-one
44. 19-norpregn-5(10)-en-20-yn-3-one, 17-hydroxy-, (17alpha)-
45. 17-alpha-ethynyl-5(10)-estren-17-ol-3-one
46. Cas-68-23-5
47. 17alpha-ethinyl-5(10)estraen-17beta-ol-3-one
48. Ncgc00016304-01
49. 17alpha-ethynyl-estr-5(10)-en-3-on-17beta-ol
50. Prestwick_883
51. 17-alpha-ethynyl-17-hydroxyestr-5(10)-en-3-one
52. 17-alpha-ethynyl-estr-5(10)-en-3-on-17-beta-ol
53. 17alpha-ethinyl-delta(sup)-5,10-19-nortestosterone
54. 17alpha-ethynyl-17beta-hydroxyestr-5(10)-en-3-one
55. Estr-5(10)-en-3-one, 17alpha-ethynyl-17-hydroxy-
56. 17-alpha-ethinyl-delta(super)-5,10-19-nortestosterone
57. 17-beta-hydroxy-17-alpha-ethinyl-5(10)-estren-3-one
58. 17-hydroxy(17alpha)-19-norpregn-5(10)-en-20-yn-3-one
59. 17-hydroxy-19-nor-17alpha-pregn-5(10)-en-20-yn-3-one
60. 17alpha-ethynyl-19-nor-5(10)-androsten-17beta-ol-3-one
61. (17-alpha)-17-hydroxy-19-norpregn-5(10)-en-20-yn-3-one
62. 17-alpha-ethynyl-19-nor-5(10)-androsten-17-beta-ol-3-one
63. 17alpha-ethinyl-17beta-hydroxy-delta(sup 5(10))-estren-3-one
64. 17alpha-ethinyl-19-nor-delta-5(10)-androstene-17beta-ol-3one
65. 17alpha-ethynyl-17beta-hydroxy-delta(sup 5(10))-estren-3-one
66. 19-nor-17alpha-pregn-5(10)-en-20-yn-3-one, 17-hydroxy-
67. 17-alpha-ethinyl-17-beta-hydroxy-delta(sup 5(10))-estren-3-one
68. 17-alpha-ethynyl-17-beta-hydroxy-3-oxo-delta(sup 5(10))-estrene
69. 17-alpha-ethynyl-17-beta-hydroxy-delta(sup. 5(10))-estren-3-one
70. 17alpha-ethynyl-17beta-hydroxy-3-oxo-delta(sup 5(10))-estrene
71. Dsstox_cid_563
72. Opera_id_1052
73. Prestwick0_000024
74. Prestwick1_000024
75. Prestwick2_000024
76. Prestwick3_000024
77. 17-.alpha.-ethynyl-17-hydroxyestr-5(10)-en-3-one
78. 17.alpha.-ethinyl-.delta.5(10)-19-nortestosterone
79. Norethynodrel [mi]
80. 17-.alpha.-ethinyl-.delta.5(10)-19-nortestosterone
81. 17.alpha.-hydroxy-19-norpregn-5(10)-en-20-yn-3-one
82. 5(10)-estren-3-one, 17alpha-ethynyl-17beta-hydroxy-
83. 17.alpha.-ethinyl-.delta.(sup)-5,10-19-nortestosterone
84. Chembl1387
85. Dsstox_rid_75927
86. Norethynodrel [hsdb]
87. Dsstox_gsid_21069
88. Schembl37838
89. Bspbio_000087
90. Norethynodrel [vandf]
91. Noretynodrel [mart.]
92. 13-methyl-17-ethynyl-17-hydroxy-1,2,3,4,6,7,8,9,11,12,13,14,16,17-tetradecahydro-15h-cyclopenta(a)-phenanthrene-3-one
93. 17-.alpha.-ethynyl-17-.beta.-hydroxy-5(10)-estren-3-one
94. 17-.beta.-hydroxy-17-.alpha.-ethinyl-5(10)-estren-3-one
95. Bidd:er0192
96. Noretynodrel [who-dd]
97. Spbio_002008
98. (17-.alpha.)-17-hydroxy-19-norpregn-5(10)-en-20-yn-3-one
99. 17-.alpha.-ethynyl-17-.beta.-hydroxy-.delta.5(10)-estren-3-one
100. 17.alpha.-ethinyl-17.beta.-hydroxy-.delta.5(10)-estren-3-one
101. 17.alpha.-ethynyl-17.beta.-hydroxy-.delta.5(10)-estren-3-one
102. 17.alpha.-ethynyl-17.beta.-hydroxy-3-oxo-.delta.5(10)-estrene
103. Bpbio1_000097
104. Dtxsid3021069
105. 17-ethynyl-17-hydroxyestr-.delta.5(10)-en-3-one, (17.alpha.,17.beta.)-
106. Hms1568e09
107. Hms2095e09
108. Hms2232j10
109. Hms3712e09
110. Norethynodrel [orange Book]
111. 19-nor-17-alpha-pregn-5(10)-en-20-yn-3-one, 17-hydroxy-
112. Hy-b1341
113. Norethynodrel [usp Impurity]
114. Tox21_110359
115. Bdbm50410507
116. Enovid Component Norethynodrel
117. Akos026749810
118. Zinc118913164
119. Ccg-220024
120. Db09371
121. Norethynodrel Component Of Enovid
122. Ncgc00179666-01
123. Cs-0013090
124. D05207
125. Norethisterone Impurity D [ep Impurity]
126. Wln: L E5 B666ov Mutj E1 F1uu1 Qq
127. (17beta-ethynyl-17-hydroxyestr-5(10)-en-3-one
128. Sr-01000721945
129. Q5659069
130. Sr-01000721945-2
131. Brd-k80334323-001-03-5
132. Estr-5(10)-en-3-one,17.alpha.-ethynyl-17-hydroxy-
133. 17-hydroxy-19-nor-17alpha-pregn-5(10)en-20-yn-3-one
134. (1r,3as,3br,9bs,11as)-1-ethynyl-1-hydroxy-11a-methyl-1h,2h,3h,3ah,3bh,4h,5h,6h,7h,8h,9h,9bh,10h,11h,11ah-cyclopenta[a]phenanthren-7-one
135. 13-methyl-17-ethynyl-17-hydroxy-1,2,3,4,6,7,8,9,11,12,13,14,16,17-tetradecahydro-15h-cyclopenta(a)phenanthren-3-one
| Molecular Weight | 298.4 g/mol |
|---|---|
| Molecular Formula | C20H26O2 |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 298.193280068 g/mol |
| Monoisotopic Mass | 298.193280068 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 606 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Contraceptives, Oral, Synthetic
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Norethynodrel indicated for the prevention of pregnancy in women who elect to use oral contraceptives as a method of contraception. /Former/
Physicians Desk Reference. 42nd ed. Thomson PDR. Montvale, NJ 1988.
Norethynodrel is a progestogen derived from 19-nortestosterone. It is used in oral contraceptive agents and hormonal pregnancy tests (no longer available in the United States).
Briggs, G.G, R.K. Freeman, S.J. Yaffe. A Reference Guide to Fetal and Neonatal Risk. Drugs in Pregnancy and Lactation. 4th ed. Baltimore, MD: Williams & Wilkins 1994., p. 635
EXPTL USE VET: Experimentally, it has been used with mestranol in swine to synchronize estrus after treatment is withdrawn. Anabolic, androgenic, and fetal masculinization effects are less than with norethindrone...
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 394
Norethynodrel exhibits a dose dependent suppression of lactation. Lower infant weight gain, decreased milk production, and decreased composition of nitrogen and protein content of human milk have been associated with similar synthetic progestogens and estrogen products ... . The magnitude of these changes is low. However, the changes in milk production and composition may be of nutritional importance in malnourished mothers. If breast feeding is desired, the lowest dose of oral contraceptives should be chosen. Monitoring of infant weight gain and the possible need for nutritional supplementation should be considered.
Briggs, G.G, R.K. Freeman, S.J. Yaffe. A Reference Guide to Fetal and Neonatal Risk. Drugs in Pregnancy and Lactation. 4th ed. Baltimore, MD: Williams & Wilkins 1994., p. 635
Use of oral contraceptives is associated with an increased risk of several serious conditions including thromboembolism, stroke, myocardial infarction, liver tumor, gallbladder disease, visual disturbances, fetal abnormalities, and hypertension. Cigarette smoking increases the risk of serious adverse cardiovascular effects during oral contraceptive use. This risk increases with age and with heavy smoking (15 or more cigarettes daily) and is markedly greater in women older than 35 years of age. Women who are receiving estrogen-progestin contraceptives should be strongly advised not to smoke. Women older than 35 years of age who smoke, and women with ischemic heart disease or a history of this disease, should not use estrogen-progestin contraceptives. Estrogen-progestin contraceptives should be used with caution in women with cardiovascular disease risk factors. /Estrogen-Progestin Combination/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3112
The most frequent adverse effect of oral contraceptives is nausea. In addition, nausea has been reported in women using vaginal or transdermal estrogen-progestin contraceptives. The principal risk associated with currently recommended high-dose, postcoital estrogen-progestin combination regimens appears to be moderate to severe adverse GI effects including severe vomiting and nausea, which occur in 12-22 and 30-66%, respectively, of women receiving the short-course regimens and may limit compliance with, and effectiveness of, the regimens. In 2 prospective, randomized studies, nausea and vomiting were less common with a high-dose postcoital progestin-only regimen (0.75 mg levonorgestrel every 12 hours for 2 doses) than with a high-dose estrogen-progestin regimen (100 mcg ethinyl estradiol and 0.5 mg levonorgestrel every 12 hours for 2 doses). Other adverse GI effects include vomiting, abdominal cramps, abdominal pain, bloating, diarrhea, and constipation. Gingivitis and dry socket have also been reported. Changes in appetite and changes in weight also may occur. /Estrogen-Progestin Combination/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3109
The most frequent dermatologic reaction to oral contraceptives is chloasma or melasma. Women who have had melasma during pregnancy appear to be most susceptible. Irregular brown macules may develop slowly on the face within 1 month to 2 years following initiation of oral contraceptive therapy. The macules fade more slowly than in melasma gravidarum and may be permanent. Acne may improve during oral contraceptive therapy because of decreased sebum production and depression of sebaceous gland activity; however, it may increase in severity during initial therapy and may develop in some women who have not previously had acne. Other dermatologic reactions include allergic rash, urticaria, erythema multiforme, erythema nodosum, hemorrhagic eruption, and pruritus. Hirsutism and alopecia have also occurred. Herpes gestationis and porphyria cutanea have reportedly been adversely affected in women receiving oral contraceptives. /Estrogen-Progestin Combination/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3109
For more Drug Warnings (Complete) data for NORETHYNODREL (32 total), please visit the HSDB record page.
3. 3= MODERATELY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) IS 0.5-5 G/KG, BETWEEN 1 OUNCE & 1 PINT (OR 1 LB) FOR 70 KG PERSON (150 LB). /ENOVID, ETHYNODREL & MESTRANOL MIXT/
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-174
Contraceptives, Oral, Hormonal
Oral contraceptives which owe their effectiveness to hormonal preparations. (See all compounds classified as Contraceptives, Oral, Hormonal.)
Contraceptives, Oral, Synthetic
Oral contraceptives which owe their effectiveness to synthetic preparations. (See all compounds classified as Contraceptives, Oral, Synthetic.)
...Metabolized in women; 75% of an acute oral dose was excreted in 7-day urine and feces, with complex pattern of urinary metabolites.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 333
It is generally considered that progestogens that are structurally related to norethisterone are pro-drugs and that their progestational activity is due to their conversion to norethisterone. After oral administration, norethisterone acetate and ethynodiol diacetate are rapidly converted to norethisterone by esterases during hepatic first-pass metabolism. Although less is known about the transformation of lynestrenol and norethynodrel, it appears that lynestrenol first undergoes hydroxylation at carbon 3 and then oxidation of the hydroxyl group to form norethisterone. Although there is no convincing evidence for the in-vivo transformation of norethynodrel to norethisterone, data from receptor binding tests and bioassays suggest that norethynodrel is also a pro-drug.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V91 146 (2007)
The /metabolic/ products /of norethynodrel/ are eliminated as glucuronides and sulfates...
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V6 39 (1974)
...Metabolic studies of norethynodrel in women have revealed the presence of the two 3-hydroxy epimers. The recovery of the two...was not large due to extensive further metabolism by routes such as hydroxylation, but keto reduction appears as the major initial pathway.
Testa, B. and P. Jenner. Drug Metabolism: Chemical & Biochemical Aspects. New York: Marcel Dekker, Inc., 1976., p. 118
Double-bond reduction is an important pathway for ... norethynodrel ... /gives/ rise to stereoisomers of 17alpha-ethynyl-5-estrane-3,17beta-diol as significant metabolites in women.
Testa, B. and P. Jenner. Drug Metabolism: Chemical & Biochemical Aspects. New York: Marcel Dekker, Inc., 1976., p. 121
From less polar fractions, there were isolated 17alpha- ethynylestr-5(10)-ene-3alpha,17beta-diol, 17alpha-ethynylestr-5 (10)-ene-3beta,17beta-diol, 17 alpha-ethynyl-5beta-estrane-3alpha,17beta-diol, and 17alpha-ethynyl-5 alpha-estrane-3beta, 17 beta-diol.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 333
Estrogen-progestin combinations produce a contraceptive effect mainly by suppressing the hypothalamic-pituitary system resulting in prevention of ovulation. The estrogen acts mainly by suppressing secretion of follicle-stimulating hormone (FSH), resulting in prevention of follicular development and the rise of plasma estradiol concentration which is thought to be the stimulus for release of luteinizing hormone (LH). In combination products, the progestin appears to act mainly by inhibiting the preovulatory rise of LH. Long-term administration of these combination products results in inhibition of both FSH and LH secretion. It has been suggested that oral contraceptives may also produce a direct effect on ovarian steroidogenesis or the response of the ovary to gonadotropins. In addition, changes in the cervical mucus may prevent sperm penetration; however, further studies are required to determine the precise effects of estrogen-progestin combinations on sperm activity.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3115
The cellular action of steroid hormones is mediated by specific receptors. ... Two different estrogen receptors (ER), alpha and beta, have been cloned with a specific tissue distribution. Active estrogen as well as active progestin are compounds of oral hormonal contraceptives and hormone replacement therapy. To examine the regulation of ER-alpha and -beta activities after treatment with synthetic progestins and synthetic and natural estrogens, COS 7 cells were transfected with the vector expressing ER-alpha and -beta in combination with a luciferase reporter vector. ER-alpha activity was upregulated in the presence of synthetic progestins in a dose-dependent manner. Norethisterone, norethynodrel and desogestrel proved to be the most potent stimulatory agents of ER-alpha expression. On the other hand, not all progestins exhibited a stimulatory action on ER-beta activity. Only norgestrel, levonorgestrel, norethynodrel and norethisterone induced ER-beta-activating functions in a dose-dependent manner. Luciferase activity due to estrogen stimulation served as a positive control. These results indicate that progestins have different effects on the activities of ER-alpha and -beta.
PMID:10836199 Rabe T et al; Gynecol Endocrinol 14 (2): 118-26 (2000)
Vascular endothelial growth factor (VEGF) is a potent angiogenic factor associated with the degree of vascularity, progression, and metastasis of breast cancer, and cases of this disease with increased vascular density have a poor prognosis. We show that in T47-D human breast cancer cells, progesterone induces a dose-dependent increase of 3-4-fold in media VEGF levels, with a maximum response occurring at a concentration of 10 nM. This effect is blocked by the antiprogestin RU 486. In addition to progesterone, a number of synthetic progestins used in oral contraceptives (eg, norethindrone, norgestrel, and norethynodrel), hormone replacement therapy (medroxyprogesterone acetate), and high-dose progestin treatment of breast cancer (megestrol acetate) also increase VEGF in the media of cultured T47-D cells. This effect is hormone specific and is not produced by estrogens, androgens, or glucocorticoids. Collectively, these observations suggest that the increase in VEGF caused by progestins is mediated by progesterone receptors present in T47-D cells. The induction of VEGF by progestins is also cell type specific and does not occur in human breast cancer cell lines MCF-7, ZR-75, or MDA-MB-231, nor in Ishikawa cells derived from a human endometrial carcinoma. ...
PMID:9458078 Hyder SM et al; Cancer Res 58 (3): 392-5 (1998)
Platelet-activating factor acetylhydrolase (PAF-AH), the enzyme that inactivates PAF, is regulated by steroid hormones including progestin. It has been reported that 17alpha-ethynylestradiol decreases plasma PAF-AH activity and medroxyprogesterone increases the enzyme activity. In this study, /investigators/ elucidated the effects of various progestins on plasma PAF-AH activity and lipoprotein cholesterol levels. Plasma PAF-AH activity in female adult rats treated with either progesterone or 17alpha-hydroxyprogesterone (50 mg/kg, 3 days) did not change significantly. Both medroxyprogesterone and megestrol acetate (50 mg/kg, 3 days) significantly increased plasma PAF-AH activity, but both norethindrone acetate and norethynodrel (50 mg/kg, 3 days) significantly decreased the enzyme activity. In addition, not only did medroxyprogesterone increase plasma PAF-AH activity but plasma lipoprotein cholesterol and norethindrone acetate decreased both of them. A significant correlation between plasma PAF-AH activity and the lipoprotein level was found (r = 0.974, p < 0.01). When PAF (10 nmol/kg) was administered to female adult rats pretreated with progestins, the mortality of the rats that had low plasma PAF-AH activity due to norethindrone acetate was increased in the same manner as observed in the 17alpha-ethynylestradiol-pretreated group. These findings indicate that progestins have various effects on plasma PAF-AH activity and the lipoprotein level. In addition, some progestins, which have an estrogenic effect on enzyme activity, may be related to the thrombotic episodes observed in oral contraceptive users.
PMID:7608617 Fujikami F et al; Nippon Sanka Fujinka Gakkai Zasshi 47 (6): 539-46 (1995)
For more Mechanism of Action (Complete) data for NORETHYNODREL (6 total), please visit the HSDB record page.