

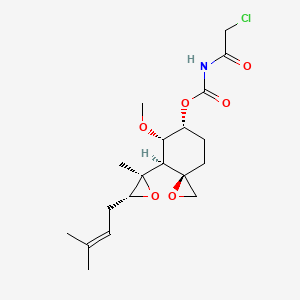

1. 5-methoxy-4-(2-methyl-3-(3-methyl-2-butenyl)oxiranyl)-1-oxaspiro(2,5)oct-6-yl(chloroacetyl) Carbamate
2. Agm 1470
3. Agm-1470
4. Agm1470
5. Caplostatin
6. Tnp 470
7. Tnp-470
8. Tnp470
1. Tnp-470
2. 129298-91-5
3. Agm-1470
4. Tnp 470
5. Agm 1470
6. O-chloroacetylcarbamoylfumagillol
7. Drg-0148
8. Nsc642492
9. Nsc-642492
10. Chembl424278
11. (3r,4s,5s,6r)-5-methoxy-4-((2r,3r)-2-methyl-3-(3-methylbut-2-en-1-yl)oxiran-2-yl)-1-oxaspiro[2.5]octan-6-yl (2-chloroacetyl)carbamate
12. Chebi:90748
13. X47gr46481
14. Carbamic Acid, N-(2-chloroacetyl)-, (3r,4s,5s,6r)-5-methoxy-4-[(2r,3r)-2-methyl-3-(3-methyl-2-buten-1-yl)-2-oxiranyl]-1-oxaspiro[2.5]oct-6-yl Ester
15. (3r,4s,5s,6r)-5-methoxy-4-[(2r,3r)-2-methyl-3-(3-methylbut-2-en-1-yl)oxiran-2-yl]-1-oxaspiro[2.5]octan-6-yl (chloroacetyl)carbamate
16. Nsc 642492
17. Ccris 8049
18. Unii-x47gr46481
19. Carbamic Acid, N-(2-chloroacetyl)-, (3r,4s,5s,6r)-5-methoxy-4-((2r,3r)-2-methyl-3-(3-methyl-2-buten-1-yl)-2-oxiranyl)-1-oxaspiro(2.5)oct-6-yl Ester
20. Schembl1652694
21. Dtxsid0041141
22. Bdbm17446
23. Tnp-470 [mi]
24. Zinc3914617
25. Ccg-208103
26. Cs-8106
27. Db08633
28. (3r,4s,5s,6r)-5-methoxy-4-[(2r,3r)-2-methyl-3-(3-methylbut-2-en-1-yl)oxiran-2-yl]-1-oxaspiro[2.5]octan-6-yl N-(2-chloroacetyl)carbamate
29. [(3r,6r,7s,8s)-7-methoxy-8-[(2r,3r)-2-methyl-3-(3-methylbut-2-enyl)oxiran-2-yl]-2-oxaspiro[2.5]octan-6-yl] N-(2-chloroacetyl)carbamate
30. Nci60_014346
31. Agm-1470; Nsc 642492
32. Hy-101932
33. Tnp-470, >=98% (hplc)
34. 298t915
35. J-005665
36. Brd-k53597484-001-01-9
37. Q27097825
38. (chloroacetyl)carbamic Acid (3r,4s,5s,5r)-5-methoxy-4-[(2r,3r)-2-methyl-3-(3-methyl-2-butenyl)oxiranyl]-1-oxaspiro[2.5]oct-6-yl Ester
39. [(3r,4s,5s,6r)-5-methoxy-4-[(2r,3r)-2-methyl-3-(3-methylbut-2-enyl)oxiran-2-yl]-1-oxaspiro[2.5]octan-6-yl] N-(2-chloroacetyl)carbamate
40. Carbamic Acid, (3r,4s,5s,6r)-5-methoxy-4-[ (2r,3r)-2-methyl-3-(3-methyl-2-butenyl)oxiranyl]-1-oxaspiro[2.5]oct-6-yl Ester
41. Carbamic Acid, (chloroacetyl)-, (3r,4s,5s,6r)-5-methoxy-4-[(2r,3r)-2-methyl-3-(3-methyl-2-butenyl)oxiranyl]-1-oxaspiro[2.5]oct-6-yl Ester
42. Carbamic Acid, (chloroacetyl)-, 5-methoxy-4-(2-methyl-3-(3-methyl-2-butenyl)oxiranyl)-1-oxaspiro(2.5)oct-6-yl Ester, (3r-(3alpha,4alpha(2r*,3r*),5beta,6beta))-
| Molecular Weight | 401.9 g/mol |
|---|---|
| Molecular Formula | C19H28ClNO6 |
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 7 |
| Exact Mass | 401.1605153 g/mol |
| Monoisotopic Mass | 401.1605153 g/mol |
| Topological Polar Surface Area | 89.7 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 636 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Angiogenesis Inhibitors
Agents and endogenous substances that antagonize or inhibit the development of new blood vessels. (See all compounds classified as Angiogenesis Inhibitors.)
Antibiotics, Antineoplastic
Chemical substances, produced by microorganisms, inhibiting or preventing the proliferation of neoplasms. (See all compounds classified as Antibiotics, Antineoplastic.)